Abstract
Though dose-response curves for large scale radiation injury to tissues are undoubtedly related to survival curves for clonogenic cells, the relationship between the two sets of curves is not necessarily simple. Sterilization of clonogenic cells occurs near-instantaneously by comparison with the protracted lag period for gross injury to tissues. Moreover, with some types of macroscopic damage, the shapes of the dose-response curves may depend on time of assay. Changes in the area or volume of irradiated tissue may also influence the shapes of these curves. The temporal pattern of expression of large scale injury also varies between tissues, and two distinct groups can be recognized. In rapidly proliferating tissues, the lag period is almost independent of dose, whilst in slowly proliferating tissues, it is inversely proportional to dose. This might be explained by invoking differences in corresponding proliferative structures of the tissues (Three compartmental Type H versus One compartmental Type F proliferative organization). For the second group of tissues, in particular, mathematical modelling suggests a systematic dissociation of the dose-response curves for clonogenic cell survival and for large scale injury. This dissociation, which arises even in the case of single doses, may be even more important when radiation is fractionated. In particular, it may be difficult to disentangle the contributions made to inter-fraction sparing by cellular repair processes and by proliferation-related factors.
Full text
PDF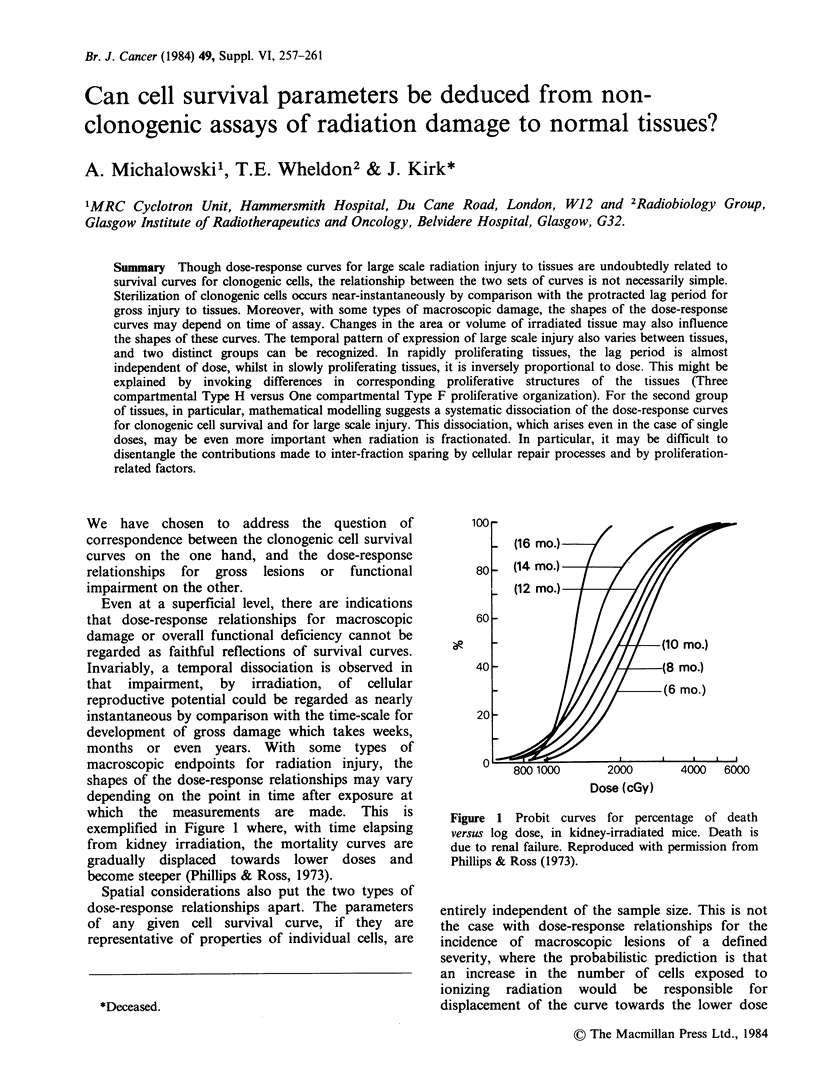


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Barwari S. E., Potten C. S. A cell kinetic model to explain the time of appearance of skin reaction after X-rays or ultraviolet light irradiation. Cell Tissue Kinet. 1979 May;12(3):281–289. doi: 10.1111/j.1365-2184.1979.tb00150.x. [DOI] [PubMed] [Google Scholar]
- Collis C. H., Steel G. G. Dose-dependence of the time of appearance of lung damage in mice given thoracic irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1982 Sep;42(3):245–252. doi: 10.1080/09553008214551171. [DOI] [PubMed] [Google Scholar]
- Mahler P. A., Oberley T. D., Yatvin M. B. Histologic examination of the influence of dietary protein on rat radiation nephropathy. Radiat Res. 1982 Mar;89(3):546–558. [PubMed] [Google Scholar]
- PATT H. M., QUASTLER H. Radiation effects on cell renewal and related systems. Physiol Rev. 1963 Jul;43:357–396. doi: 10.1152/physrev.1963.43.3.357. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., MARCUS P. I. Action of x-rays on mammalian cells. J Exp Med. 1956 May 1;103(5):653–666. doi: 10.1084/jem.103.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T. L., Ross G. A quantitative technique for measuring renal damage after irradiation. Radiology. 1973 Nov;109(2):457–462. doi: 10.1148/109.2.457. [DOI] [PubMed] [Google Scholar]
- Siemann D. W., Hill R. P., Penney D. P. Early and late pulmonary toxicity in mice evaluated 180 and 420 days following localized lung irradiation. Radiat Res. 1982 Feb;89(2):396–407. [PubMed] [Google Scholar]
- VOGEL H. H., Jr, CLARK J. W., JORDAN D. L. Comparative mortality following single whole-body exposures of mice to fission neutrons and Co60 gamma rays. Radiology. 1957 Mar;68(3):386–398. doi: 10.1148/68.3.386. [DOI] [PubMed] [Google Scholar]
- Wheldon T. E., Michalowski A. S., Kirk J. The effect of irradiation on function in self-renewing normal tissues with differing proliferative organisation. Br J Radiol. 1982 Oct;55(658):759–766. doi: 10.1259/0007-1285-55-658-759. [DOI] [PubMed] [Google Scholar]



