Abstract
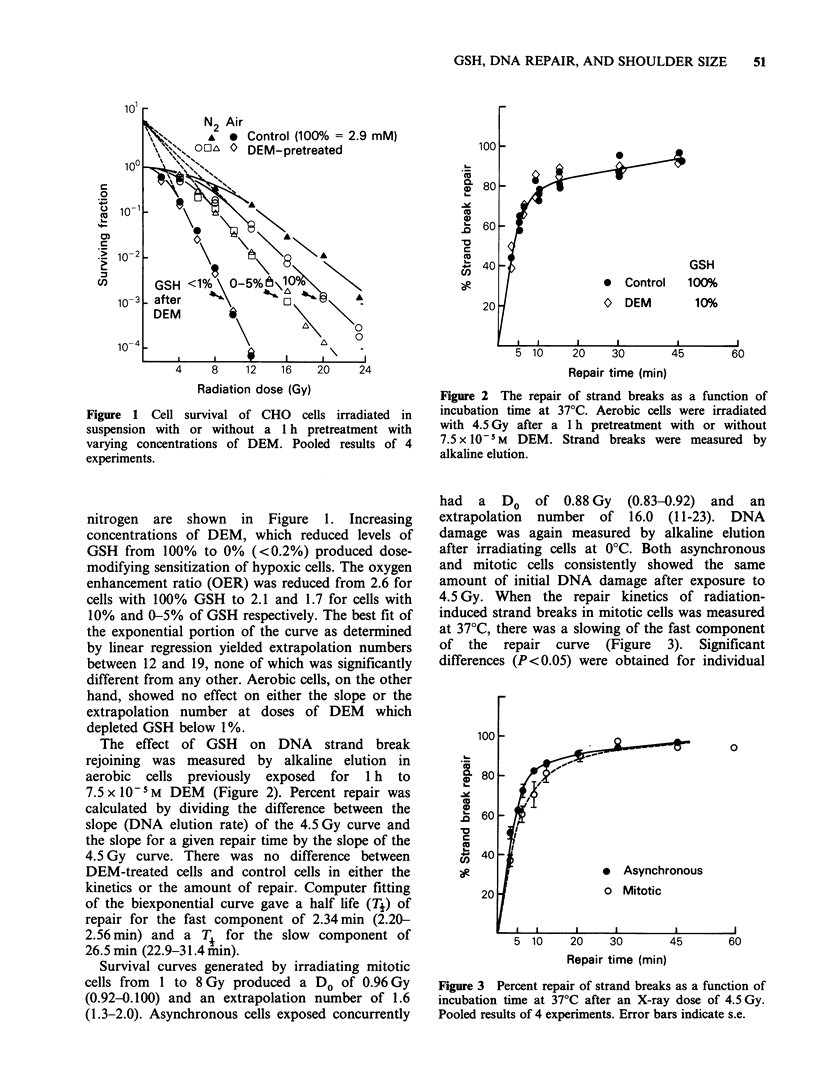
The nonprotein thiol glutathione (GSH) and the rate and extent of repair of DNA strand breaks were investigated as two possible determinants of the size of the shoulder of the X-ray survival curve in Chinese Hamster Ovary (CHO) cells. Both cell survival and DNA single strand break repair were measured at comparable radiation doses (using clonogenic and alkaline elution assays respectively) following treatment of CHO cells with agents which deplete GSH levels and/or reduce the shoulder of the radiation survival curve. CHO cells were treated with diethylmaleate (DEM) at doses which reduce GSH levels from 10% to less than 1% of control values. GSH depletion produced dose-modifying radiosensitization of hypoxic but not aerobic cells, reducing the oxygen enhancement ratio (OER) from 2.6 to 1.7, with no change in the survival curve shoulder, nor in the rate of repair of DNA strand breaks. Since mitotic CHO cells exhibited a reduced shoulder compared to asynchronous cells with no difference in the Do of the survival curve, they provided an opportunity to test the possible association of the shoulder size with the repair rate of DNA breaks. Both mitotic and asynchronous cells had the same initial number of strand breaks for a given radiation dose, but mitotic cells had a half-life for repair of those breaks which was significantly longer.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Durand R. E., Olive P. L. Radiation-induced DNA damage in V79 spheroids and monolayers. Radiat Res. 1979 Apr;78(1):50–60. [PubMed] [Google Scholar]
- Edgren M. Intercellular co-operation in repairing radiation-induced single-strand DNA breaks. Int J Radiat Biol Relat Stud Phys Chem Med. 1982 May;41(5):589–593. doi: 10.1080/09553008214550681. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P. Effect of oxygen on the radiosensitivity of bacteriophage in the presence of sulphydryl compounds. Nature. 1960 May 7;186:485–487. doi: 10.1038/186485a0. [DOI] [PubMed] [Google Scholar]
- Hall E. J., Biaglow J. Ro-07-0582 as a radiosensitizer and cytotoxic agent. Int J Radiat Oncol Biol Phys. 1977 May-Jun;2(5-6):521–530. doi: 10.1016/0360-3016(77)90163-8. [DOI] [PubMed] [Google Scholar]
- Han A., Sinclair W. K., Kimler B. F. The effect of N-ethylmaleimide on the response to x rays of synchronized HeLa cells. Radiat Res. 1976 Feb;65(2):337–350. [PubMed] [Google Scholar]
- Harris J. W., Power J. A. Diamide: a new radiosensitizer for anoxic cells. Radiat Res. 1973 Oct;56(1):97–109. [PubMed] [Google Scholar]
- Kimler B. F., Sinclair W. K., Elkind M. M. N-ethylmaleimide sensitization of X-irradiated hypoxic Chinese hamster cells. Radiat Res. 1977 Jul;71(1):204–213. [PubMed] [Google Scholar]
- Kohn K. W., Erickson L. C., Ewig R. A., Friedman C. A. Fractionation of DNA from mammalian cells by alkaline elution. Biochemistry. 1976 Oct 19;15(21):4629–4637. doi: 10.1021/bi00666a013. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Kerrigan D., Pommier Y. Inhibitors of poly-(adenosine diphosphoribose) synthesis slow the resealing rate of x-ray-induced DNA strand breaks. Biochem Biophys Res Commun. 1982 Feb 11;104(3):897–902. doi: 10.1016/0006-291x(82)91333-x. [DOI] [PubMed] [Google Scholar]



