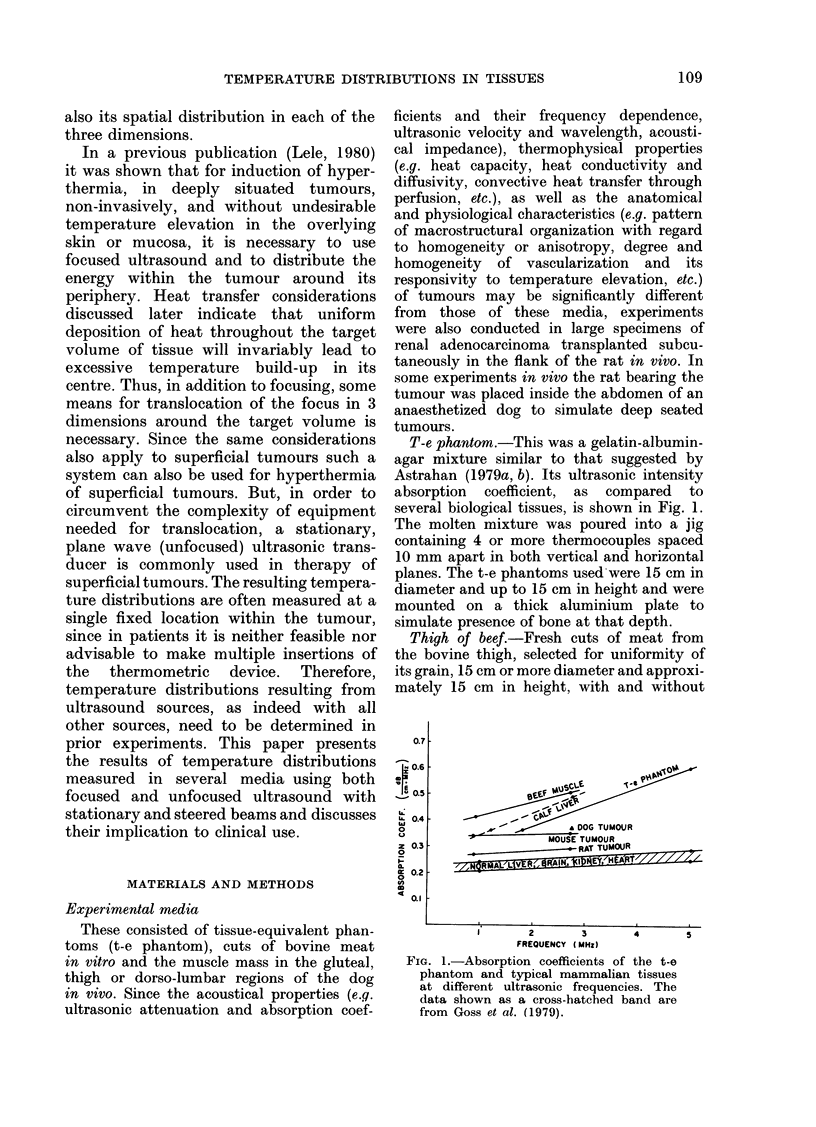
Abstract
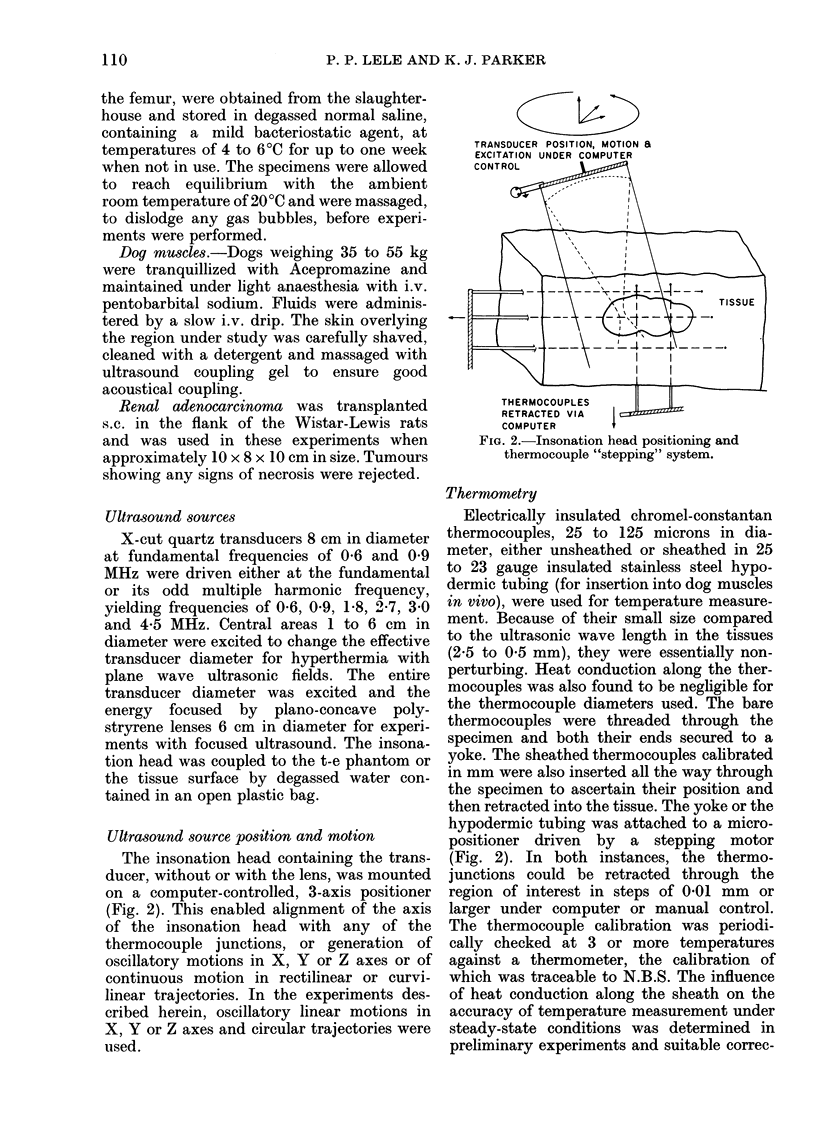
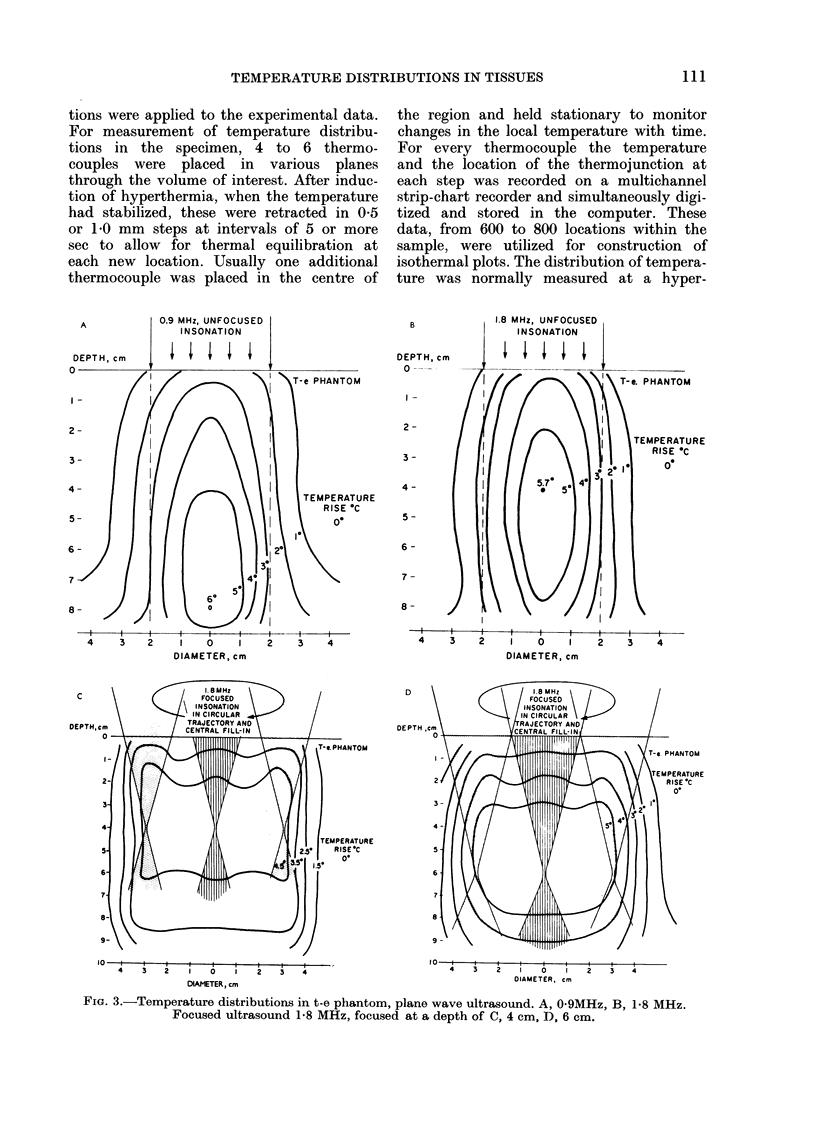
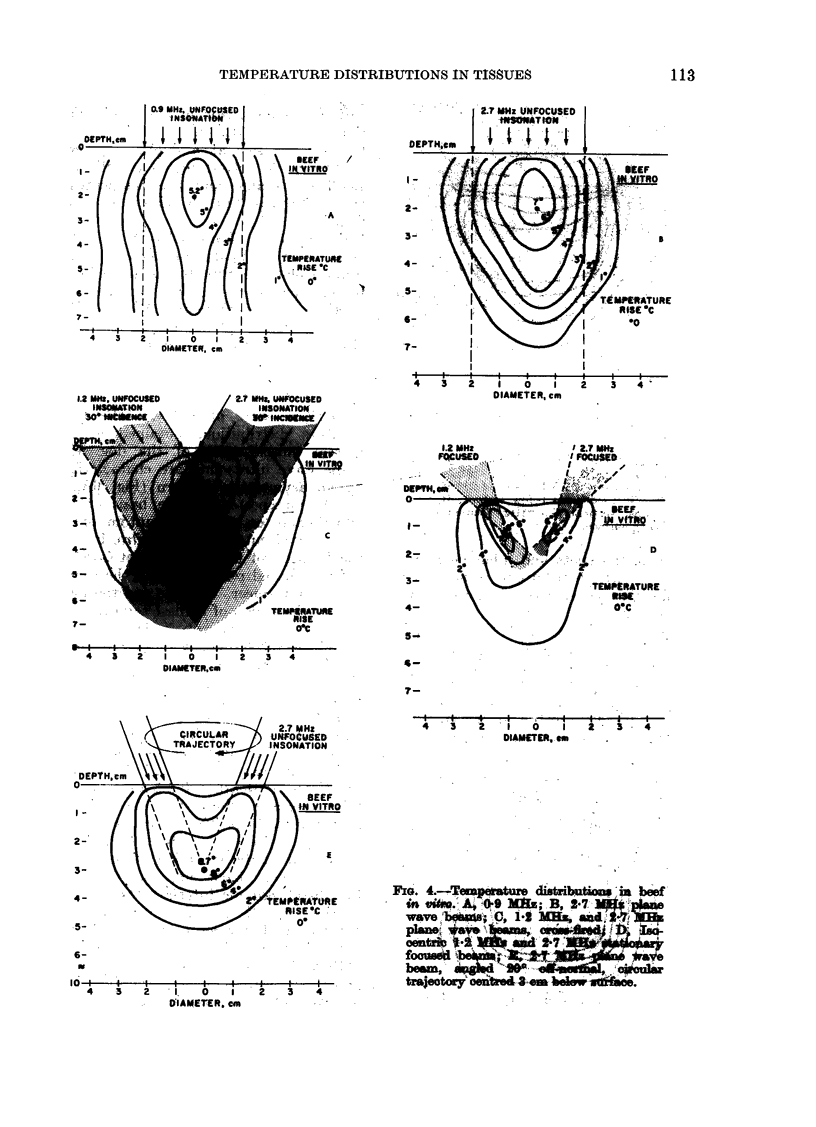
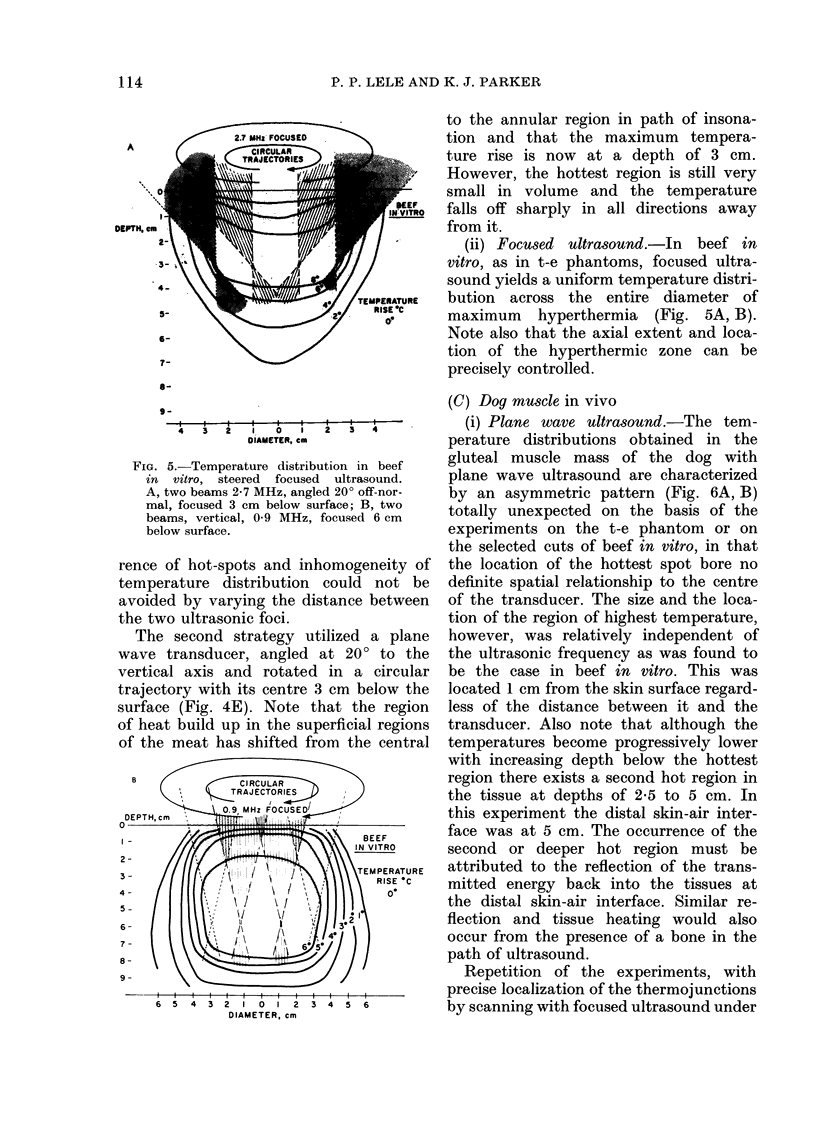
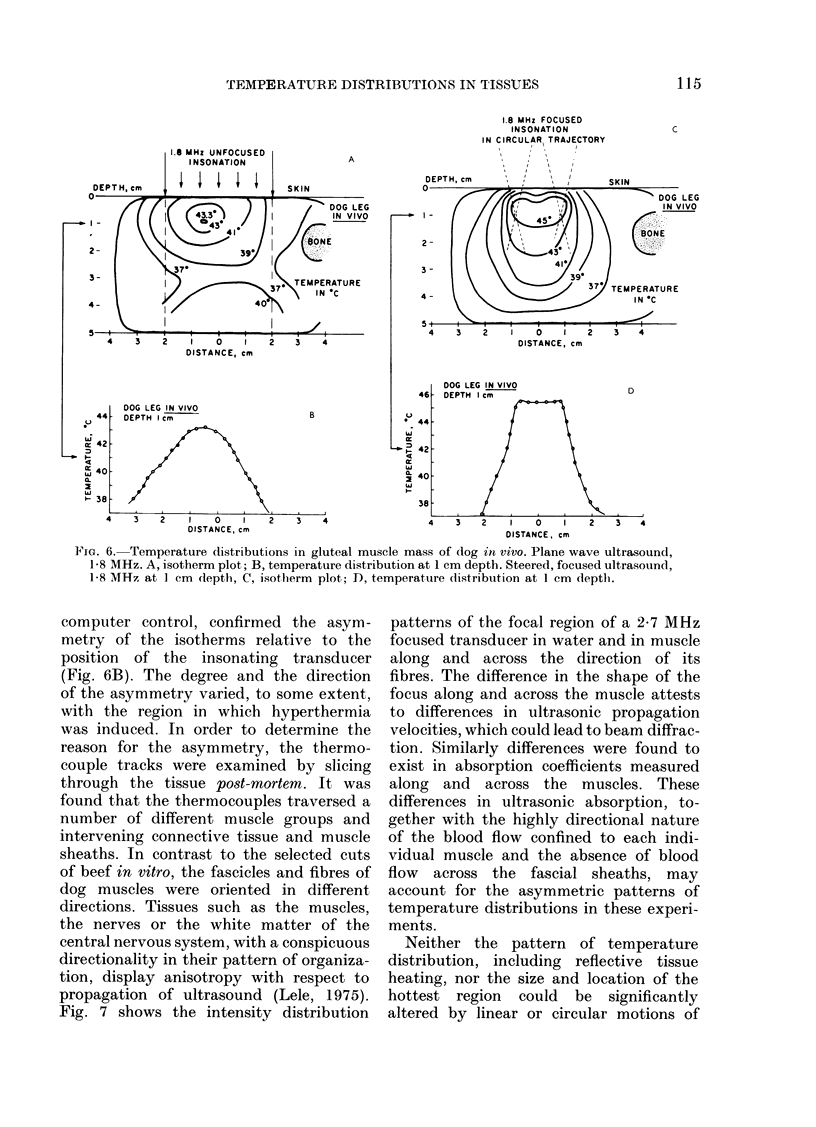
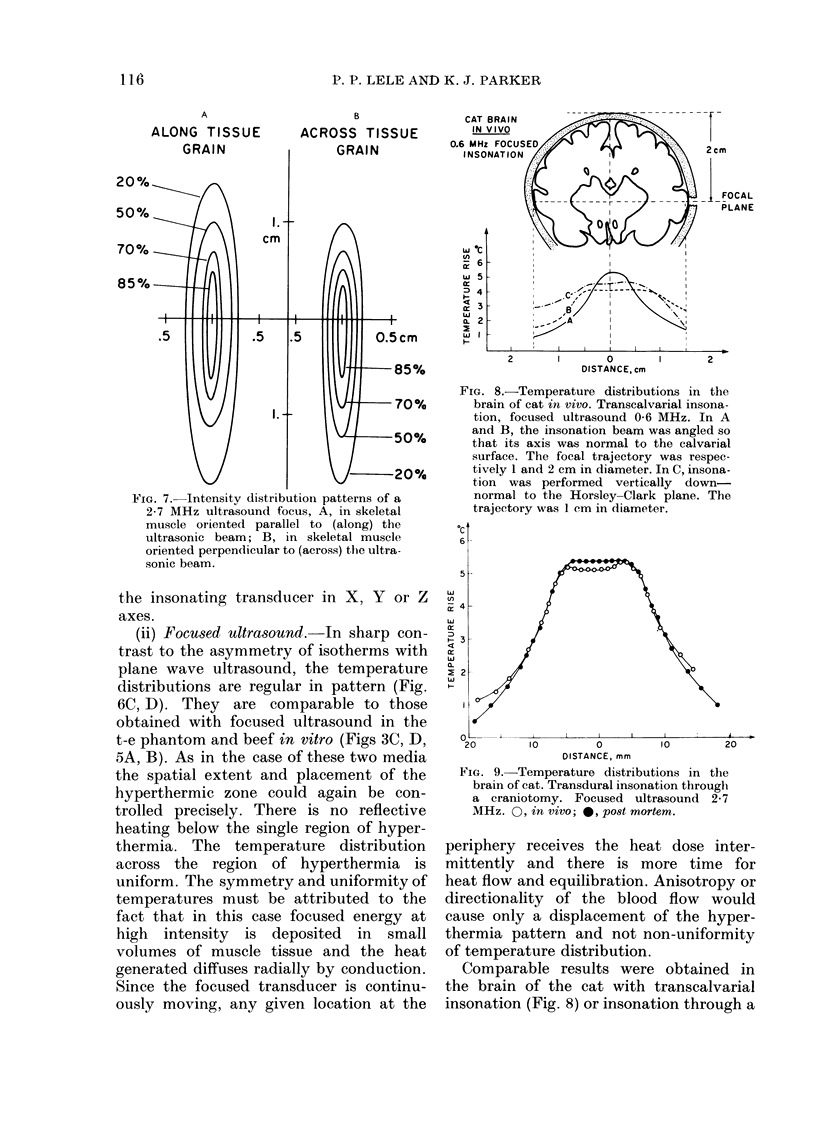
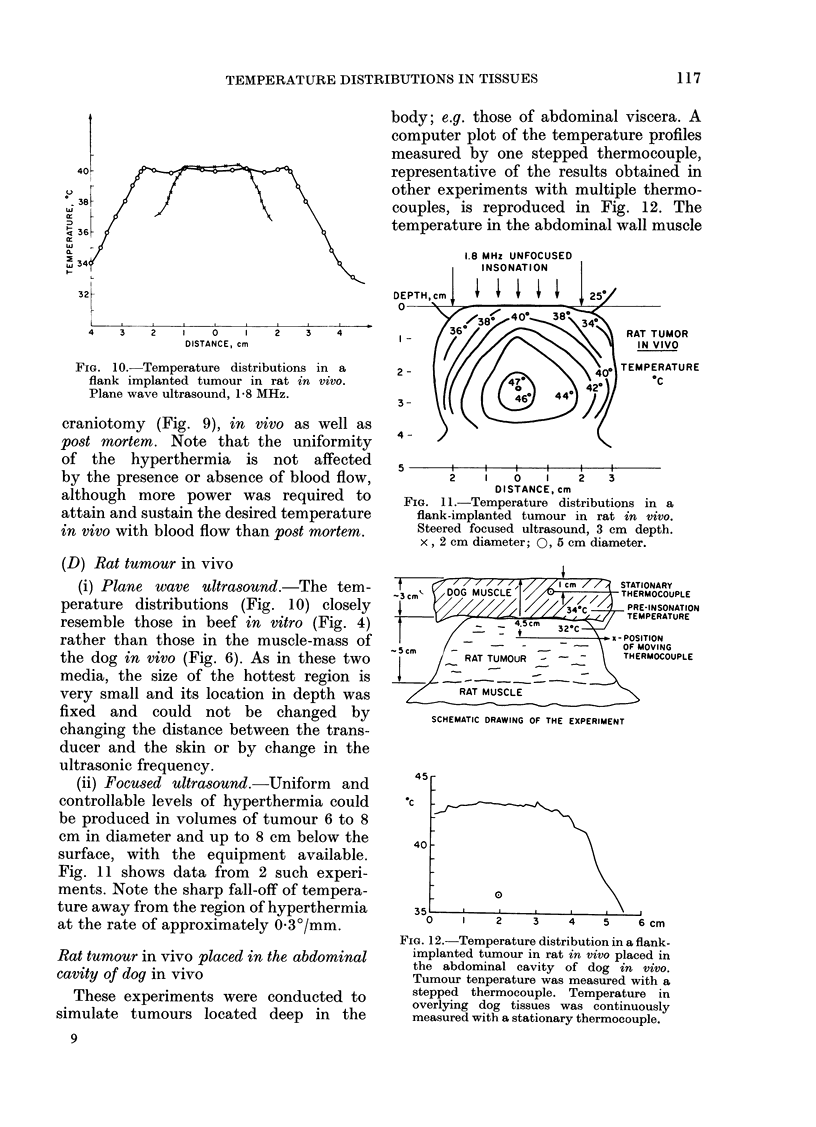
Temperature distributions resulting from insonation with stationary or steered beams of unfocused or focused ultrasound were measured in tissue-equivalent phantom, beef muscle in vitro, dog muscle mass, and transplanted murine tumours in vivo. Arrays of 4 to 6 thermocouples stepped through the volume of interest under computer control were used to measure the steady-state temperatures at 600 to 800 locations in both in vitro and in vivo experiments. The results were confirmed in spontaneous tumours in dog patients using fewer multi-thermocouple probes. Plane wave ultrasound was found to result in spatially non-uniform hyperthermia even in superficial tumours. The region of maximum temperature rise was small in extent and was situated at a depth which varied in the different models from 0.5 to 1.0 cm. Neither its location nor its extent could be varied by spatial manipulations of the transducer or by changing the insonation parameters except the ultrasonic frequency. A second region of hyperthermia was produced at depth by reflective heating if an ultrasonically reflective target, such as bone or air-containing tissue, was located below the target tissue. On the other hand, using available steered, focused ultrasound techniques, tumours (whether situated superficially or at depth) could be heated to a uniform, controllable temperature without undesirable temperature elevation in surrounding normal tissues. The use of steered, focused ultrasound permits deposition of energy to be tailored to the specific needs of each individual tumour. The small size of the focal region enables heating of tumours even when located near ultrasound reflecting targets.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrahan M. A. Hyperthermia phantom. Med Phys. 1979 Jan-Feb;6(1):72–72. doi: 10.1118/1.594523. [DOI] [PubMed] [Google Scholar]
- Goss S. A., Frizzell L. A., Dunn F. Ultrasonic absorption and attenuation in mammalian tissues. Ultrasound Med Biol. 1979;5(2):181–186. doi: 10.1016/0301-5629(79)90086-3. [DOI] [PubMed] [Google Scholar]
- Lele P. P. A strategy for localized chemotherapy of tumors using ultrasonic hyperthermia. Ultrasound Med Biol. 1979;5(1):95–96. doi: 10.1016/0301-5629(79)90134-0. [DOI] [PubMed] [Google Scholar]
- Lele P. P. Induction of deep, local hyperthermia by ultrasound and electromagnetic fields: problems and choices. Radiat Environ Biophys. 1980;17(3):205–217. doi: 10.1007/BF01323647. [DOI] [PubMed] [Google Scholar]
- Marmor J. B., Hilerio F. J., Hahn G. M. Tumor eradication and cell survival after localized hyperthermia induced by ultrasound. Cancer Res. 1979 Jun;39(6 Pt 1):2166–2171. [PubMed] [Google Scholar]
- Marmor J. B., Pounds D., Hahn N., Hahn G. M. Treating spontaneous tumors in dogs and cats by ultrasound-induced hyperthermia. Int J Radiat Oncol Biol Phys. 1978 Nov-Dec;4(11-12):967–973. doi: 10.1016/0360-3016(78)90007-x. [DOI] [PubMed] [Google Scholar]
- Marmor J. B., Pounds D., Postic T. B., Hahn G. M. Treatment of superficial human neoplasms by local hyperthermia induced by ultrasound. Cancer. 1979 Jan;43(1):188–197. doi: 10.1002/1097-0142(197901)43:1<188::aid-cncr2820430128>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]


