Abstract
Embryonic development requires cell migration in response to positional cues. Yet, how groups of cells recognize and translate positional information into morphogenetic movement remains poorly understood. In the developing kidney, the ureteric bud epithelium grows from the nephric duct towards a group of posterior intermediate mesodermal cells, the metanephric mesenchyme, and induces the formation of the adult kidney. The secreted protein GDNF and its receptor RET are required for ureteric bud outgrowth and subsequent branching. However, it is unclear whether the GDNF–RET pathway regulates cell migration, proliferation, survival, or chemotaxis. In this report, we have used the MDCK renal epithelial cell line to show that activation of the RET pathway results in increased cell motility, dissociation of cell adhesion, and the migration towards a localized source of GDNF. Cellular responses to RET activation include the formation of lamellipodia, filopodia, and reorganization of the actin cytoskeleton. These data demonstrate that GDNF is a chemoattractant for RET-expressing epithelial cells and thus account for the developmental defects observed in RET and GDNF mutant mice. Furthermore, the RET-transfected MDCK cells described in this report are a promising model for delineating RET signaling pathways in the renal epithelial cell lineage.
Keywords: GDNF, RET, kidney development, chemoattraction, MDCK cells
During embryonic development, secreted signaling molecules can specify the fates of adjacent cells, stimulate or inhibit cellular proliferation, and act as attractants or repellents for axonal guidance. The developing kidney is a well-studied paradigm for signaling interactions between epithelial and mesenchymal tissues (Saxen 1987; Lechner and Dressler, 1997). The kidney, or metanephros, becomes induced when the ureteric bud, an outgrowth of the nephric duct epithelium, contacts the metanephric mesenchyme, a group of posterior, intermediate mesodermal cells that are already predetermined to form renal epithelium. Classical tissue recombination experiments had demonstrated that the ureteric bud sends permissive signals to promote aggregation of the mesenchyme and phenotypic conversion to renal epithelium (Grobstein 1955, 1956). Thus, much of the tubular epithelium of the nephron is derived from mesenchymal cells. Reciprocally, the metanephric mesenchyme emits signals that instruct the ureteric bud to proliferate and undergo branching morphogenesis, to induce new nephrons along the radial axis of the growing kidney, and to generate the collecting duct system.
The receptor tyrosine kinase RET is an essential component of the signaling pathway which regulates ureteric bud outgrowth and branching. RET is expressed along the nephric duct and in the newly formed ureteric bud, but becomes restricted to the growing tips of the bud as branching morphogenesis progresses (Pachnis et al., 1993; Avantaggiato et al., 1994). Homozygous RET-null mice exhibit renal agenesis due in large part to an inhibition of ureteric bud outgrowth (Schuchardt et al., 1994, 1996). In a low percentage of mice, the ureteric bud is formed but branching is inhibited. Strikingly, the metanephric mesenchyme of RET-null mice is still able to respond to inductive signals (Schuchardt et al., 1996). A similar renal phenotype was observed in homozygous mice carrying a null allele of the gene encoding glial cell-derived neurotrophic factor (GDNF)1 (Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996). GDNF expression is localized to the metanephric mesenchyme at early stages and is repressed in the induced mesenchyme as epithelial conversion occurs (Hellmich et al., 1996). However, GDNF remains restricted to mesenchyme in the peripheral nephrogenic zone as the kidney develops. GDNF is able to stimulate branching morphogenesis in the kidney (Vega et al., 1996) and is sufficient to induce ectopic ureteric buds from the nephric duct (Sainio et al., 1997). Moreover, GDNF can activate the RET tyrosine kinase domain (Trupp et al., 1996; Vega et al., 1996). However, binding of GDNF to RET and activation of the catalytic domain requires a high affinity, GPI-linked coreceptor, GFRα-1 (Jing et al., 1996; Treanor et al., 1996; Sanicola et al., 1997), which is expressed in both mesenchyme and ureteric bud epithelium (Sainio et al., 1997).
In the embryonic kidney, RET-expressing ureteric bud epithelial cells (Pachnis et al., 1993) grow towards the metanephric mesenchyme, which expresses GDNF (Hellmich et al., 1996). In the gut, RET-expressing neural crest cells migrate into the developing gut endoderm, also expressing GDNF, to generate the enteric neurons of the intestinal tract. How activation of RET affects the ureteric bud epithelium to promote directional growth remains obscure. Localized proliferation and physical constraints could push the growing bud out of the existing duct. Differences in the composition of extracellular matrix in the metanephric mesenchyme, relative to surrounding mesoderm, could provide for a favorable environment for bud extension in response to growth factors. Alternatively, the GDNF– RET pathway could provide and translate positional guidance cues for migrating ureteric bud epithelial cells.
The growing tip of the ureteric bud epithelium is a highly specialized tissue that expresses, in addition to RET, a number of other markers, such as wnt-11 (Kispert et al., 1996) and c-ros (Tessarollo et al., 1992), not found in the more mature stalk of the epithelium. Unfortunately, immortalized cell lines that correspond this growth zone of the bud have not been identified. In this report, we have used the established renal cell line MDCK to examine the effects of RET activation on cell morphology, motility, and migratory potential. The MDCK cell is morphologically most like renal distal tubular epithelium (Leighton et al., 1969; Rindler et al., 1979) and has retained the ability to form tubules in three-dimensional culture systems (Montesano et al., 1991). In RET-transfected MDCK-derived clones, RET could be activated in a ligand-independent manner by overexpressing the protein or in a ligand-dependent manner by the addition of GDNF and soluble GFRα-1. Activation of RET resulted in cell scattering, increased cell motility, and cell migration towards a localized source of GDNF. These effects are accompanied by changes in cellular morphology, including the redistribution of actin stress fibers and focal adhesion molecules and the formation of lamellipodia and filopodia. These data support the hypothesis that GDNF is a target-derived guidance cue for epithelial cell migration into the metanephric mesenchyme.
Materials and Methods
Cell Lines
MDCK cells, clone 3B5, were cultured in a 5% CO2/95% air environment with DME containing 10% fetal bovine serum. Subconfluent cells in 10-cm dishes were transfected with pCMVRET-HA/neo construct using lipofectamine (Life Technologies, Inc., Gaithersburg, MD) reagent as described by the manufacturer. Stable lines were selected in 400 μg/ml G418. The kinase mutant was constructed by site-directed mutagenesis which converts lysine 758 to methionine (Liu et al., 1996). To observe RET activation, cells were incubated with DME containing 10 or 1% serum for 3 d before immunoprecipitation. Alternatively, subconfluent cells were cultured in 10% serum supplemented with 100 ng/ml of soluble, recombinant human GFRα-1 (previously known as GDNFR-α or RETL1) in the presence or absence of 50 ng/ml recombinant human GDNF (Promega Corp., Madison, WI) for a specified time from 5 min to 24 h. RET immunoprecipitation and Western blotting with anti-phosphotyrosine (pTyr) and anti-HA antibodies was done as described previously (Vega et al., 1996). Anti–E-cadherin staining was done as described (Ryan et al., 1995; Cho et al., 1998). After fixing in 4% paraformaldehyde for 10 min, and rinsing in PBS/0.1% Tween-20, cells were stained with FITC-phalloidin (1 μg/ml, Sigma Chemical Co., St. Louis, MO) in PBS/0.1% Tween-20/2% goat serum. Focal adhesion complexes were stained using anti-vinculin mouse monoclonal antibody (Sigma Chemical Co.) at a dilution of 1:400 and a Texas red-conjugated goat anti–mouse secondary antibody (Molecular Probes, Inc., Eugene, OR) at 1:200.
Cell proliferation assays were done on either plastic-coated dishes or on thin collagen gels, in the presence or absence of GDNF/GFRα-1. To examine the effects of RET activation by ligand, 10,000 cells were plated in six-well dishes at day 0 and counted every 2 d. Three independent experiments were averaged and the standard error calculated.
Cell Migration Assays
A modified Boyden chamber assay was used to assess cell migration through filters. To evaluate the migration potential, RET-2, RET-9, and KM cells were cultured with 10 or 1% serum for 3 or 5 d. Cells were washed, trypsinized, and then cell numbers were counted under a hemocytometer. For ligand-dependent cell migration, 105 cells were plated onto the polyethylene terephthalate (PET) filters containing 8-μm pores (Falcon Plastics, Cockeysville, MD) with media supplemented with 10% serum and 100 ng/ml of GFRα-1. GDNF was added to the bottom chamber or both the bottom and top chambers at either 10 or 50 ng/ml. After a 24-h incubation, cells on top of the filters were scraped and the remaining cells on the bottom side of the filter were fixed, stained, and then counted under the light microscope. Five fields were counted at 100× and the average number of cells and standard deviation from the mean were calculated. Each condition was repeated with at least three independent experiments. The KM cells did not migrate across the filter under any of the experimental conditions used.
Chemoattraction Assays
Preparation of type I collagen gel has been described by Montesano et al. (1991). A six-well plate was coated with 0.5 ml of collagen gel (Collaborative Biomedical Products, Waltham, MA) per well. Heparin−acrylamide beads (Sigma Chemical Co.) were washed in PBS and incubated with 10 ng/μl recombinant GDNF (Promega Corp.) or BSA for 1 h. Beads were washed in PBS and then inserted in type I collagen gel before the gel solidified. The collagen gel helps to maintain the chemical gradient of the ligands in their local microenvironment created by the beads. Ret9 and KM cells were seeded on the surface of the gel at 20,000 cells/well with media containing 10% serum and 100 ng/ml of soluble GFRα-1. Cells around the beads were photographed over time at 8-h intervals.
Organ Culture
Kidneys were cultured and stained as described (Vega et al., 1996). DME supplemented with 10% serum was used in all organ cultures. GDNF and BSA beads were prepared as described above. Kidney rudiments were dissected from 11.5-d-old embryos, at the time when the ureteric bud had formed a T-shaped structure. A single bead was placed at one side of the embryonic kidney. After 2 d, kidneys were fixed in cold methanol for 10 min, washed in PBST (PBS containing 0.1% Tween 20), and then stained with a pancytokeratin monoclonal (Sigma Chemical Co.) to visualize the ureteric bud branching pattern.
Results
To develop a model system for examining the effects of RET activation on renal epithelial cells, the MDCK cell line was transfected with expression vectors encoding either the human wild-type RET or a kinase-deficient mutant form (KM), both containing a hemagglutinin (HA) epitope tag at the carboxyl terminus (Liu et al., 1996). The MDCK cells have been used extensively as a model epithelial cell type and are well characterized with respect to their origin from the distal part of the nephron (Rindler et al., 1979). Furthermore, MDCK cells are able to form epithelial cysts and tubules in three-dimensional collagen gels (Montesano et al., 1991). Multiple RET-expressing cell lines were identified by Western blotting using an anti-HA antibody. The wild-type RET-expressing MDCK clonal lines used for this study were Ret2 and Ret9, where Ret9 expressed four- or fivefold more protein than Ret2 (data not shown). The cell line KM expressed the kinase mutant form of RET at levels comparable to Ret2.
RET Activation Promotes Scattering but Not Proliferation
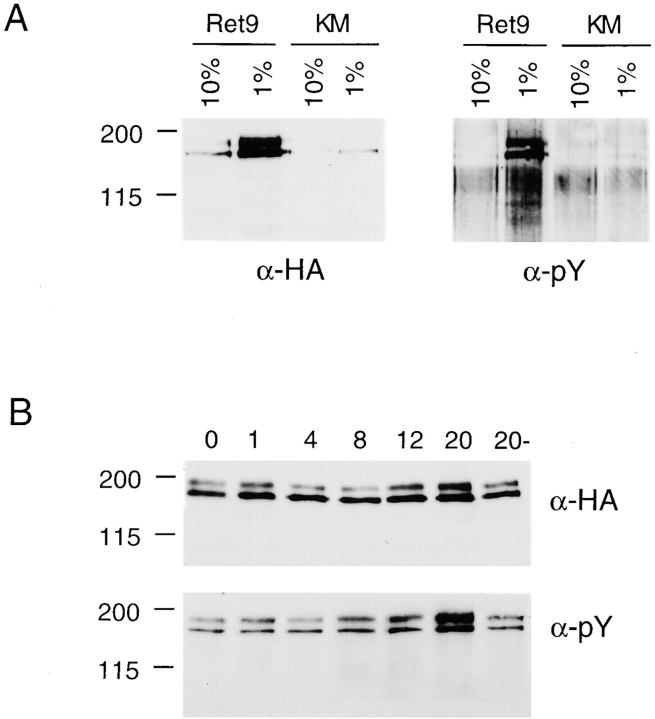
Initial experiments designed to characterize the growth properties of the Ret2, Ret9, and KM cells in either 10 or 1% serum revealed a high level of RET activation upon prolonged growth in 1% serum (Fig. 1 A). Phosphorylation of RET was assayed by immunoprecipitation with anti-HA followed by Western blotting with an anti-pTyr antibody. Phosphorylation of the larger 170-kD protein is indicative of RET activation. Under normal culture conditions in 10% fetal calf serum, Ret9 cells had a low basal level of phosphorylated RET (Fig. 1 A). However, when the same cells were cultured in 1% serum, the level of activated RET increased dramatically as indicated by high levels of the 170-kD phosphorylated RET protein in the Ret9 cells but not in the KM cells. Northern blot analysis indicated a three- to fivefold increase in RET mRNA driven from the cytomegalovirus (CMV) promoter (data not shown) and Western blotting using anti-HA or anti-RET antibodies indicated increased amounts of RET protein (Fig. 1 A). Ret2 and Ret9 cells were able to autoactivate RET in low serum, most probably due to increased expression from the CMV promoter used in the expression vectors and subsequent autodimerization of the RET protein.
Figure 1.
(A) Autophosphorylation of RET in response to low serum. Ret9 and KM cells were cultured in 10 or 1% serum and then RET was immunoprecipitated with anti-HA and detected with anti-HA or anti-pTyr. (B) Ligand-dependent phosphorylation of RET. Ret9 cells cultured in 10% serum and 100 ng/ml GFRα-1 were exposed to 50 ng/ml GDNF for the indicated times in hours; the control culture 20− did not receive GFRα-1 or GDNF.
To activate RET in a more controlled, ligand-dependent manner, both GDNF and soluble GFRα-1 were required. Neither Ret2 nor Ret9 cells activate the RET protein in response to GDNF alone since they do not express the necessary coreceptor protein, GFRα-1. However, when soluble, recombinant GFRα-1 was added together with GDNF, and RET phosphorylation was observed in Ret9 cells cultured in 10% serum (Fig. 1 B). Maximum activation of RET was observed after 20 h of GDNF/GFRα-1 treatment. Although it is not entirely clear why activation was not observed sooner, this extended lag period may reflect the kinetics of GDNF/GFRα-1 association in solution, rather than at the cell surface where the GPI-linked GFRα-1 is usually located.
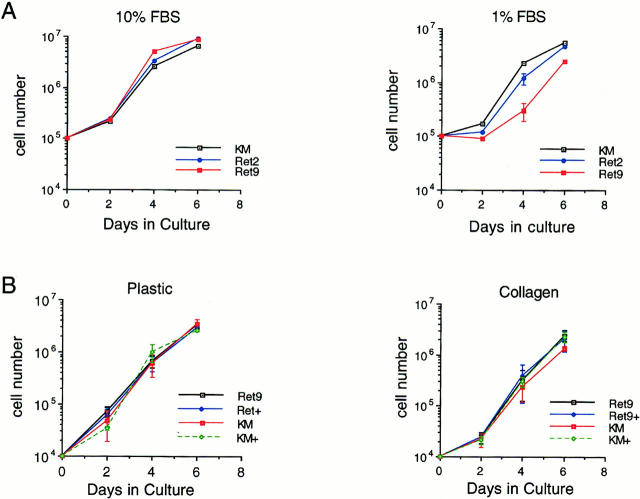
To determine the effects of RET activation on proliferation, growth curves were determined in 10 and 1% serum and in response to ligands on various culture surfaces (Fig. 2). In 10% serum, Ret9, Ret2, and the KM cells grew at similar rates (Fig. 2 A). In 1% serum, the Ret9 cells that exhibited the highest amounts of phosphorylated RET did not proliferate any faster than Ret2 or KM cells. The parental MDCK grew at the same rate as the KM cells (data not shown). If anything, Ret9 cells exhibited a slight decrease in cell number, relative to controls, after 3 d in culture upon RET activation in low serum. Activation of RET by exogenous ligands also did not significantly increase cell proliferation (Fig. 2 B). On plastic tissue culture dishes, there was no increase in growth of Ret9 cells when cultured in the presence of GDNF/GFRα-1. On the surface of collagen gels, no significant differences on proliferation of Ret9 cells were observed upon GDNF/GFRα-1 treatment after 4 or 6 d. Given that the doubling time of the MDCK-derived lines is ∼12 h, even relatively small differences in proliferation rates should become apparent after 6 d.
Figure 2.
(A) Proliferation curves of Ret9, Ret2, and KM cells in 10 or 1% serum. Cells were seeded at 100,000 cells per 60-mm dish and counted over 6 d. Cell numbers were averaged from three independent experiments. (B) Effect of GDNF/ GFRα-1 on proliferation of Ret9 and KM cells. Cells were seeded at 10,000 cells per well in six-well dishes in the presence (+) or absence of 50 ng/ml GDNF and counted every 2 d. Cells were seeded either on plastic or collagen gels as indicated. Cell numbers represent averages from three independent experiments. The error bars represent one standard deviation from the mean.
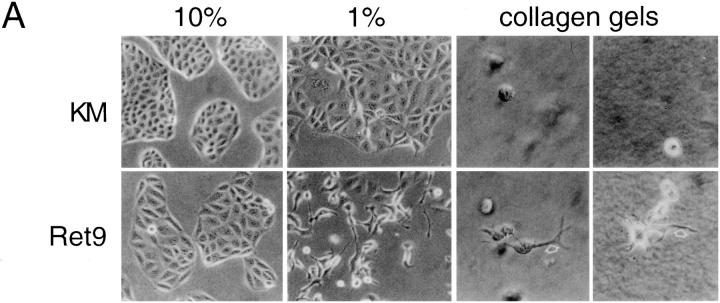
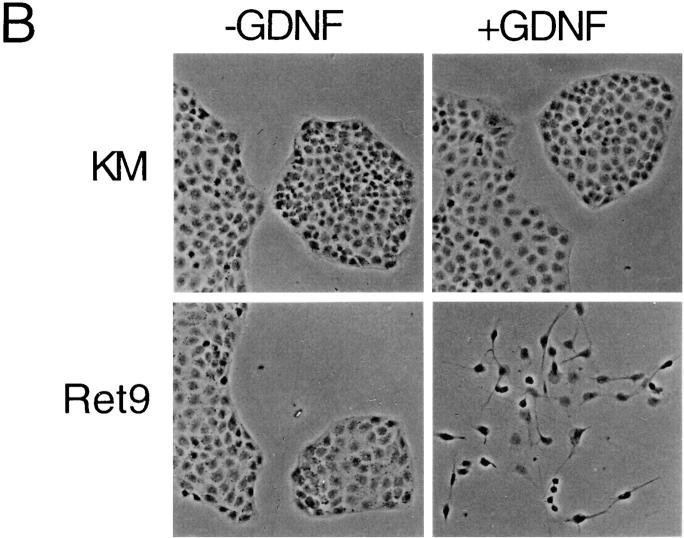
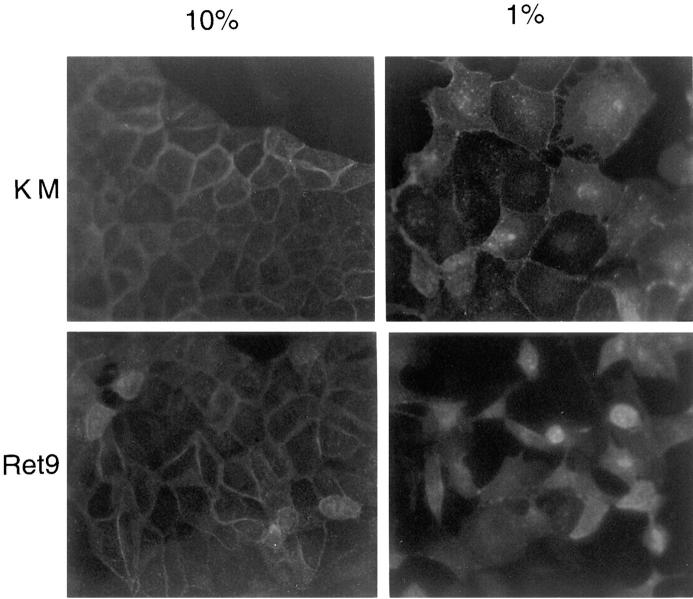
To develop biological assays for RET-expressing derivative cell lines, the Ret2, Ret9, KM, and parental MDCK cells were cultured under a variety of conditions. In high serum all cells grew as islands on plastic and exhibit tight associations (Fig. 3 A). However, in low serum or in the presence of GDNF/GFRα-1, Ret2 and Ret9 cells, but not KM cells, become dissociated and sent out long cellular processes. The cell scattering observed with activated RET could be quantified by counting the number of cells that have lost contact with their neighbors. Approximately 60% of the Ret9 cells and 12% or the Ret2 cells become scattered when cultured in 1% serum, whereas less than 2% of the KM cells exhibit scattering under similar conditions. In addition to cell scattering, RET activation in low serum promoted the outgrowth of long cellular processes, particularly evident when the cells were seeded in three-dimensional collagen gels (Fig. 3 A). Similar results were obtained when Ret9 cells were cultured with GDNF and GFRα-1 (Fig. 3 B). Cells became detached and sent out long cellular processes, particularly evident at the edges of cellular islands. KM (Fig. 3 B) and parental MDCK (data not shown) cells did not exhibit any response to GDNF/ GFRα-1. Scattering of Ret9 cells in response to RET activation was accompanied by a loss of E-cadherin staining at the cell surface (Fig. 4). In contrast, KM cells became flattened out in low serum but maintained their E-cadherin– positive junctions.
Figure 3.
Morphology of RET expressing MDCK cells. (A) Ret9 and KM cells grown in 10% serum, 1% serum, or on collagen gels. Arrows, cellular processes exclusive to Ret9 cells and clearly evident in collagen cultures. (B) Effect of GDNF/GFRα-1 on morphology of Ret9 and KM cells on plastic.
Figure 4.
Immunostaining for E-cadherin shows tight cellular junctions in KM and Ret9 cells grown in 10% serum. Ret9 cells loose E-cadherin staining in 1% serum. E-cadherin staining used rat monoclonal DECMA-1 (Sigma Chemical Co.) as described previously (Ryan et al., 1995; Cho et al., 1998).
Increased Cell Motility of RET-activated Cells
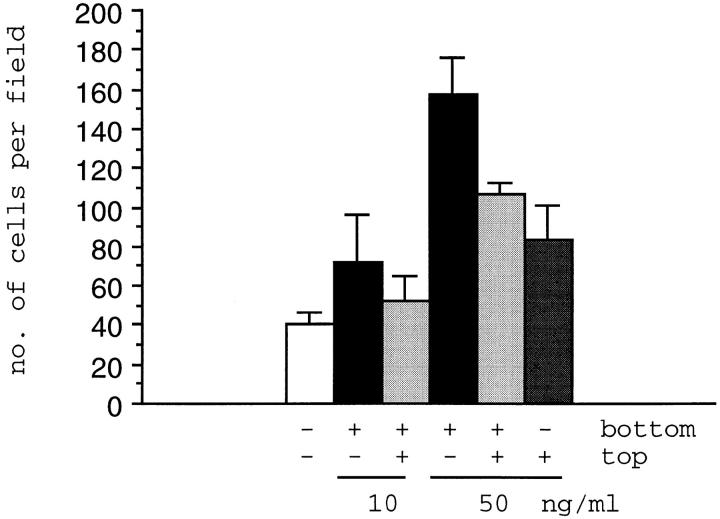
Given the increased scattering upon RET activation, the motility of Ret9 and KM cells was examined by testing their ability to migrate through a porous filter. Ret9 and KM cells were seeded on Transwell inserts containing PET filters with an 8-μm pore size, cultured in 10% serum supplemented with soluble GFRα-1, and then allowed to become confluent. Increasing amounts of GDNF were added to the underside of the filters, or bottom chamber, or to the top chamber, and the cells cultured for an additional 24 h. The number of cells migrating through the filter were scored by light microscopy (Fig. 5). The KM cells were unable to migrate through the filter under any of the conditions analyzed (data not shown). Ret9 cells were able to migrate through the filter at a low basal rate of ∼40 cells per field of view. Addition of 10 ng/ml GDNF to the bottom chamber showed a twofold increase in Ret9 cells migrating through the filter. If 10 ng/ml GDNF was added to both chambers, this twofold increase was reduced slightly. However, at 50 ng/ml of GDNF in the bottom chamber, the number of Ret9 cells migrating through was increased fourfold. This fourfold increase was significantly reduced if 50 ng/ml GDNF was added to both top and bottom chambers. Addition of 50 ng/ml GDNF to the top chamber only reduced the transfilter migration even further. The data demonstrate increased cell motility upon activation of RET. These data also suggest that transfilter migration of Ret9 cells is due, at least in part, to chemotaxis, since fewer cells were observed migrating through the filter when GDNF was present in both chambers than when GDNF was present in the bottom chamber only.
Figure 5.
Transfilter chemotaxis assay of Ret9 cells. Cells were cultured in 10% serum and 100 ng/ml GFRα-1. After confluence, 10 or 50 ng/ml of GDNF was added to one or both chambers of the transfilter insert as indicated. The average number of cells per field is shown from a representative experiment with the error bars represent one standard deviation. Experiments were done in triplicate.
Cell motility and scattering is mediated by the reorganization of the cytoskeleton. To examine reorganization of the actin filaments and focal adhesion complexes, Ret9 cells cultured in the presence or absence of GDNF/GFRα-1 were stained with FITC-phalloidin and antivinculin antibodies (Fig. 6). Control Ret9 cells contain many stress fibers running along the entire length of the cells, with particularly thick staining bundles along the periphery of the epithelial islands (Fig. 6 A). After RET activation, stress fibers are much less apparent (Fig. 6, B and D), strong actin staining is seen in the lamellipodia, and filopodia are seen all along the cell membrane (Fig. 6 E). Actin polymerization is also seen in membrane ruffles after GDNF/ GFRα-1 treatment (Fig. 6 D). Focal adhesion complexes are found at the termini of stress fibers and are randomly distributed along the untreated cells (Fig. 6 F) but become localized to the leading edges of extending lamellipodia upon RET activation (Fig. 6, G and H). These data demonstrate that RET activation results in the redistribution of focal adhesion complexes, the formation of filopodia and lamellipodia, and the reorganization of the actin cytoskeleton.
Figure 6.
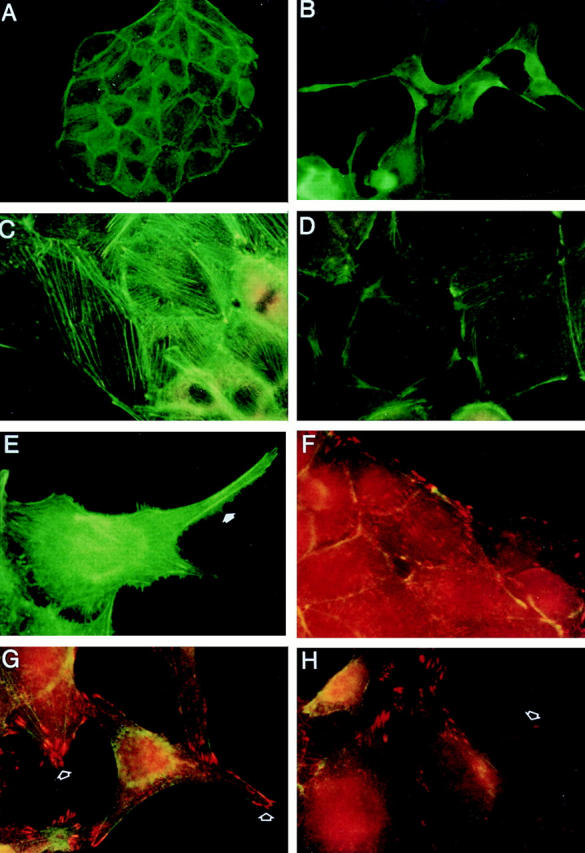
Effects of RET Activation on the actin cytoskeleton and focal adhesions. Ret9 cells cultured in media alone (A, C, and F) or with GDNF/GFRα-1 (B, D, E, G, and H). Cells were stained with FITC-phalloidin or antivinculin (red). Untreated cells exhibit prominent stress fibers (A and C), whereas RET activation results in fewer stress fibers and actin accumulation in lamellipodia (B) and at focal points along the cell membrane (D). Actin staining also reveals many filapodia (E, arrow) along the membrane of RET-activated cells. Control cells exhibit vinculin staining (F, red) at the termini of stress fibers. RET activation (G and H) results in vinculin staining at the leading edges of newly formed lamellipodia (arrows).
GDNF Is a Chemoattractant
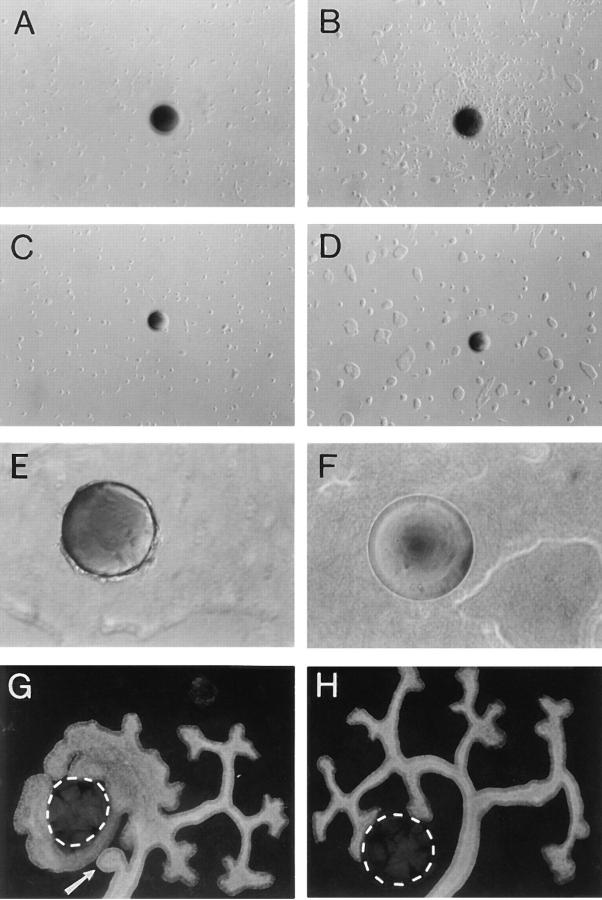
As the activation of RET increased motility, we designed experiments to examine if the RET-expressing cells could respond to and migrate towards a localized source of GDNF. Heparin−acrylamide beads were soaked in GDNF or BSA and placed in a 0.3% collagen gel that covered the bottom of the culture dish. Ret9 and KM cells were seeded on top of the gel at a low density (20,000 cells/well), supplemented with GFRα-1, and then observed over 4 d (Fig. 7). After 2 d in culture, Ret9 cells near the beads exhibited scattering and sent out processes, whereas KM cells showed no observable phenotype. After 3 d in culture on collagen gels, Ret9 cells surrounded the area of the GDNF beads (Fig. 7 B) but not the BSA control beads. After 4 d in culture, Ret9 cells could be seen attached to the GDNF beads (Fig. 7 E) but not to BSA-coated beads (Fig. 7 F). KM cells did not exhibit any migration or attraction to GDNF beads and the control BSA beads were essentially inert. Thus, Ret9 cells were able to sense local concentrations of GDNF and migrate towards their source. This attraction was dependent on the kinase activity of RET and required the GFRα-1 coreceptor. In kidney organ cultures, GDNF-soaked beads were placed on one side of an E11.5 isolated kidney rudiment and cultured for 2 d (Fig. 7 G). The ureteric bud grew towards and completely surrounded the GDNF beads. Ectopic ureteric bud branches growing from the stalk of the bud were also routinely observed (Fig. 7 G, arrow). Our results obtained with kidney organ cultures were essentially identical to the previously reported ability of GDNF to induce ectopic ureteric buds (Sainio et al., 1997). Thus, the ureteric bud epithelium could migrate towards a localized concentration of GDNF, in concordance with the Ret9 cell data.
Figure 7.
Chemoattraction assay. (A) Ret9 cells adjacent to a GDNF bead at 1 d in culture. (B) Same view as A after 3 d. (C) KM cells adjacent to a GDNF bead at 1 d in culture. (D) Same area as C after 3 d. (E) High magnification of Ret9 cells attached to a GDNF bead after 4 d in culture. (F) Ret9 cells do not attach to a BSA-coated bead after 4 d. (G) E11 kidney rudiment cultured for 2 d with a GDNF bead (outlined) at one side of the ureteric bud. Note ectopic branch coming from the stalk of the ureter (arrow), where branches are normally not seen. (H) E11 kidney cultured for 2 d with a BSA bead (outlined) at one side.
Discussion
Much of the available data regarding GDNF signaling and function has been obtained in neuronal cells where effects on differentiation and survival are pronounced (for review see Lapchak et al., 1996; Lindsay and Yancopoulos, 1996). However, in addition to neural defects, both GDNF and RET mutant mice exhibit severe renal agenesis because the ureteric bud fails to grow out of the nephric duct epithelium (Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996). Since RET signaling in the renal epithelium has not been studied in detail and cells corresponding to the ureteric bud are not readily available, we have developed RET-expressing MDCK cell lines so that the morphologic effects of RET signaling can be examined in a culture system. The MDCK cell lines are an excellent tissue culture model for a polarized renal-derived epithelium that can respond to both mitogenic and tubulogenic factors. The data presented in this report demonstrate that activation of RET in MDCK cells results in increased cell motility and migration. The RET-expressing epithelial cells are able to migrate towards a localized source of GDNF, indicating chemoattraction at the cellular level.
The ureteric bud grows out of the caudal end of the nephric duct, adjacent to the metanephric mesenchyme. Although RET is expressed along the entire length of the duct at this time (Pachnis et al., 1993), GDNF is found only in the mesenchyme (Hellmich et al., 1996; Sainio et al., 1997). In organ culture, GDNF beads can induce ectopic ureteric buds (Sainio et al., 1997), demonstrating that GDNF is sufficient for outgrowth. The data of Sainio et al. (1997), together with our own MDCK chemoattraction and migration studies, clearly demonstrate that GDNF is a guidance cue for directional growth of the ureteric bud epithelium. This interpretation is supported by genetic data from both GDNF and RET mutants. Other genes that affect the ureteric bud in a noncell autonomous manner include the Wilms's tumor suppressor gene, WT1, which is expressed in the mesenchyme, but not in the ureteric bud, yet inhibits ureteric bud outgrowth (Kreidberg et al., 1993). Thus, WT1 may be required for GDNF expression and/or maintenance of the mesenchymal cells in a viable state.
Migration of MDCK cells in response to RET activation is accompanied by the outgrowth of cellular processes, or lamellipodia, in scattered epithelial cells. These processes have also been observed in RET-transformed neuronal cells (van Weering and Bos, 1997). In addition to playing a fundamental role in cell migration (Cooper, 1991), these cellular processes might sense local concentrations of ligands and thus direct cells to their source. In fact, we have observed cellular processes extending towards and attaching to the GDNF-soaked beads in culture. All of these effects are dependent on the kinase activity of RET and correlate with the level of RET phosphorylation. The effects on cell morphology observed upon RET activation are very similar to those obtained with hepatocyte growth factor (HGF) (Ridley et al., 1995) and may be mediated, at least in part, by common signaling pathways downstream of the small GTP-binding proteins Ras, Rac, Rho, and/or Cdc42 (Tapon and Hall, 1997). In MDCK cells, the HGF-mediated rearrangement of the actin cytoskeleton and cell spreading requires Ras and Rac, whereas Rho appeared not to be required for motility (Ridley et al., 1995). Whether RET acts through any or all of these GTP-binding proteins remains to be seen.
Cell scattering has also been observed in neuroepithelioma cells upon expression and activation of an EGFR/ RET chimeric receptor (van Puijenbroek et al., 1997) and resulted in sustained activation of ERK2. Yet constitutively activate, oncogenic forms of RET, corresponding to MEN2B mutations, decrease cell adhesion and increasing metastatic potential while activating the Jun amino-terminal kinase pathway and not ERK1 or ERK2 (Marshall et al., 1997). RET signaling has been studied in some detail using chimeric and/or oncogenic forms of RET and has pointed to interactions with a variety of downstream targets, including Shc, Grb2 (Borello et al., 1994), phospholipase C-γ (Borello et al., 1996), Grb7 (Pandey et al., 1996), Grb10 (Pandey et al., 1995), and enigma (Durick et al., 1996). Yet, it is unclear how many of these potential RET-binding proteins function in migration and chemoattraction during ureteric bud growth. The MDCK cell system described in this report can now be used to assay various RET mutants in potential docking sites and help to delineating downstream RET signaling pathways specific for epithelial cells.
Activation of RET in MDCK cells does not elicit a significant mitogenic response under any of the conditions tested. Thus, the cells observed nearing the GDNF beads over time, in the chemoattraction experiments, are due to cell detachment and migration from surrounding cellular islands. In vivo, the outgrowth of the duct must also be coupled to cell proliferation. Indeed, increased cell division is observed in ureteric bud tips upon localized GDNF application (Pepicelli et al., 1997). However, MDCK cells do not proliferate in response to either ligand-dependent or -independent RET activation. In contrast, HGF stimulates both proliferation and motility in MDCK cells through the c-met receptor tyrosine kinase (for review see Gherardi and Stoker, 1991). Thus, the metanephric mesenchyme could be providing additional factors, such as HGF, to increase proliferation of ureteric bud cells and this proliferative effect is more pronounced as GDNF-dependent branching morphogenesis is stimulated. Kidney organ culture experiments also point to a role for HGF in branching morphogenesis (Santos et al., 1994; Woolf et al., 1995). However, neither HGF (Schmidt et al., 1995; Uehara et al., 1995) nor c-met (Bladt et al., 1995) homozygous mutant mice exhibit morphologically detectable ureteric bud defects.
The migration of the ureteric bud must require a precise balance between the attraction to GDNF and the retention of adhesion among epithelial cells. In our culture conditions, MDCK cells become scattered as they migrate. At first glance, this would not appear to be the case for ureteric bud epithelium, otherwise its essential epithelial character would be disturbed. However, it has been reported that there is a degree of delamination from the tips of the ureteric bud epithelium (Qiao et al., 1995), closest to the source of GDNF, and that these delaminated cells can contribute to other parts of the nephron previously thought to arise solely from the mesenchyme. Thus, the chemoattraction model can explain not only the GDNF and RET mutant phenotypes but also the recent cell lineage studies in the developing kidney. Furthermore, activating RET mutations have been associated with familial medullary thyroid carcinoma (Hofstra et al., 1994; Mulligan et al., 1994) in which parafollicular C cells delaminate from the epithelial follicle and migrate into the surrounding stroma (Ball et al., 1996). Thus, the activated RET pathway may also promote the ability of dysplastic cells to become metastatic and invade surrounding tissue.
The data presented in this report indicate several novel activities not previously associated with the GDNF–RET pathway. Activation of RET in the MDCK cell system clearly alters the adhesion and migration ability, most probably due to cytoskeletal and focal adhesion complex rearrangements and lamellipodia formation. These migrating cells are attracted to localized sources of GDNF, thus making the RET-transfected MDCK cell an excellent model for examining RET signaling and chemoattraction at the cellular level.
Acknowledgments
We thank C. Worby and J. Dixon (both from University of Michigan, Ann Arbor, MI) for the CMV–RET and CMV–KM expression constructs. This work was done during M.-J.Tang's sabbatical year in G.R. Dressler's lab. M.-J. Tang thanks N. Yanagawa whose family generously donated research funding to National Cheng Kung University Medical College in Tainan, Taiwan, without which M.-J. Tang's sabbatical would not be possible.
This work is supported by a grant from the National Institutes of Health (DK-51043) to G.R. Dressler.
Abbreviations used in this paper
- CMV
cytomegalovirus
- GDNF
glial cell-derived neurotrophic factor
- GFRα-1
GDNF family receptor α-1
- HA
hemagglutinin
- HGF
hepatocyte growth factor
- KM cells
kinase-deficient mutant cells
- pTyr
phosphotyrosine
Footnotes
Address all correspondence to G.R. Dressler, Department of Pathology, HHMI, University of Michigan, Ann Arbor, MI 48109. Tel.: (734) 764-6532. Fax: (734) 763-6640. E-mail: dressler@umich.edu
M.-J. Tang is on leave from the Department of Physiology, National Cheng Kung University Medical College, Tainan, Taiwan.
References
- Avantaggiato V, Dathan NA, Grieco M, Fabien N, Lazzaro D, Fusco A, Simeone A, Santoro M. Developmental expression of the RETprotooncogene. Cell Growth Differ. 1994;5:305–311. [PubMed] [Google Scholar]
- Ball, D.W., S.B. Baylin, and A.C. de Bustros. 1996. Medullary thyroid carcinoma. In Werner and Ingbar's The Thyroid. L.E. Braverman and R.D. Utiger, editors. Lippincott-Raven Press, Philadelphia, PA. 946–960.
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Borello MG, Pelicci G, Arighi E, De Filippis L, Greco A, Bongarzone I, Rizzetti MG, Pelicci PG, Pierotti MA. The oncogenic versions of the Ret and Trk tyrosine kinases bind Shc and Grb2 adaptor proteins. Oncogene. 1994;9:1661–1668. [PubMed] [Google Scholar]
- Borrello MG, Alberti L, Arighi E, Bongarzone I, Battistini C, Bardelli A, Pasini B, Piutti C, Rizzetti MG, Mondellini P, Radice MT, Pierotti MA. The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase cγ. Mol Cell Biol. 1996;16:2151–2163. doi: 10.1128/mcb.16.5.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR. Differential expression and function of cadherin-6 during renal epithelium development. Development (Camb) 1998;125:4806–4815. doi: 10.1242/dev.125.5.803. [DOI] [PubMed] [Google Scholar]
- Durick K, Wu R-Y, Gill GN, Taylor SS. Mitogenic signaling by Ret/ptc2 requires association with enigma via a lim domain. J Biol Chem. 1996;271:12691–12694. doi: 10.1074/jbc.271.22.12691. [DOI] [PubMed] [Google Scholar]
- Cooper JA. The role of actin polymerization in cell motility. Annu Rev Physiol. 1991;53:585–605. doi: 10.1146/annurev.ph.53.030191.003101. [DOI] [PubMed] [Google Scholar]
- Gherardi E, Stoker M. Hepatocyte growth factor-scatter factor: mitogen, motogen, and Met. Cancer Cells. 1991;3:227–232. [PubMed] [Google Scholar]
- Grobstein C. Inductive interaction in the development of the mouse metanephros. J Cell Zool. 1955;130:319–339. [Google Scholar]
- Grobstein C. Trans-filter induction of tubules in mouse metanephric mesenchyme. Exp Cell Res. 1956;10:424–440. doi: 10.1016/0014-4827(56)90016-7. [DOI] [PubMed] [Google Scholar]
- Hellmich HL, Kos L, Cho ES, Mahon KA, Zimmer A. Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mech Dev. 1996;54:95–106. doi: 10.1016/0925-4773(95)00464-5. [DOI] [PubMed] [Google Scholar]
- Hofstra RMW, Landsvater RM, Ceccherini I, Stulp RP, Stelwagen T, Luo Y, Pasini B, Hoppener JWM, Ploos van Amstel HK, Romeo G, et al. A mutation in the RETproto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367:375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Holst PL, Mei YL, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, et al. GDNF-induced activation of the Ret protein tyrosine kinase is mediated by GDNFR-a, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development (Camb) 1996;122:3627–3637. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT1is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Miller PJ, Jiao S, Araujo DM, Hilt D, Collins F. Biology of glial cell line-derived neurotrophic factor (GDNF): implications for the use of GDNF to treat Parkinson's disease. Neurodegeneration. 1996;5:197–205. doi: 10.1006/neur.1996.0027. [DOI] [PubMed] [Google Scholar]
- Lechner MS, Dressler GR. The molecular basis of embryonic kidney development. Mech Dev. 1997;62:105–120. doi: 10.1016/s0925-4773(97)00667-9. [DOI] [PubMed] [Google Scholar]
- Leighton J, Brada Z, Estes LW, Justh G. Secretory activity and oncogenicity of a cell line (MDCK) derived from canine kidney. Science. 1969;163:472–473. doi: 10.1126/science.163.3866.472. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Yancopoulos GD. GDNF in a bind with known orphan: accessory implicated in new twist. Neuron. 1996;17:571–574. doi: 10.1016/s0896-6273(00)80189-0. [DOI] [PubMed] [Google Scholar]
- Liu X, Quinn CV, Decker RA, Pandey A, Worby CA, Dixon JE. Oncogenic RET receptors display different autophosphylation sites and substrate specificities. J Biol Chem. 1996;271:5309–5312. doi: 10.1074/jbc.271.10.5309. [DOI] [PubMed] [Google Scholar]
- Marshall GM, Peaston AE, Hocker JE, Smith SA, Hansford LM, Tobias V, Norris MD, Haber M, Smith DP ,. M.J. Lorenzo, B.A.J. Ponder, and J.F. Hancock. Expression of multiple endocrine neoplasia 2B RET in neuroblastoma cells alters cell adhesion in vitro and enhances metastatic behavior in vivo, and activates jun kinase. Cancer Res. 1997;57:5399–5405. [PubMed] [Google Scholar]
- Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryans AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Mulligan LM, Eng C, Healey CS, Clayton D, Kwok JBJ, Gardner E, Ponder MA, Frilling A, Jackson CE, Lehnert H, Neumann HPH, Thibodeau SN, Ponder BAJ. Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nat Genet. 1994;6:70–74. doi: 10.1038/ng0194-70. [DOI] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development (Camb) 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Pandy A, Duan H, Di-Fiore PP, Dixit VM. The Ret receptor protein tyrosine kinase associates with the SH2-containing adapter protein Grb10. J Biol Chem. 1995;270:21461–21463. doi: 10.1074/jbc.270.37.21461. [DOI] [PubMed] [Google Scholar]
- Pandy A, Liu X, Dixon JE, Di-Fiore PP, Dixit VM. Direct association between the Ret receptor tyrosine kinase and the Src homology 2-containing adapter protein Grb7. J Biol Chem. 1996;271:10607–10610. doi: 10.1074/jbc.271.18.10607. [DOI] [PubMed] [Google Scholar]
- Pepicelli CV, Kispert A, Rowitch DH, McMahon AP. GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev Biol. 1997;192:193–198. doi: 10.1006/dbio.1997.8745. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm A-C, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Qiao J, Cohen D, Herzlinger D. The metanephric blastema differentiates into collecting system and nephron epithelia in vitro. Development (Camb) 1995;121:3207–3214. doi: 10.1242/dev.121.10.3207. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/ hepatocyte growth factor responses by ras, rac, and rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindler MJ, Chuman LM, Shaffer K, Saier MH. Retention of differentiated properties in an established cell line (MDCK) J Cell Biol. 1979;81:635–648. doi: 10.1083/jcb.81.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G, Steele-Perkins V, Morris J, Rauscher FJ, III, Dressler GR. Repression of Pax-2 by WT1 during normal kidney development. Development (Camb) 1995;121:867–875. doi: 10.1242/dev.121.3.867. [DOI] [PubMed] [Google Scholar]
- Sainio K, Suvanto P, Davies J, Wartiovaara J, Wartiovaara K, Saarma M, Arume U, Meng X, Lindahl M, Pachnis V, Sariola H. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development (Camb) 1997;124:4077–4087. doi: 10.1242/dev.124.20.4077. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Sanicola M, Hession C, Worley D, Carmillo P, Ehrenfels C, Walus L, Robinson S, Jaworski G, Wei H, Tizard R, Whitty A, Pepinsky RB, Cate RL. Glial cell-line-derived neurotrophic factor-dependent RET activation can be mediated by two different cell-surface accessory proteins. Proc Natl Acad Sci USA. 1997;94:6238–6243. doi: 10.1073/pnas.94.12.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos OFP, Barros EJG, Yang X-M, Matsumoto K, Nakamura T, Park M, Nigam SK. Involvement of hepatocyte growth factor in kidney development. Dev Biol. 1994;163:525–529. doi: 10.1006/dbio.1994.1169. [DOI] [PubMed] [Google Scholar]
- Saxen, L. 1987. Organogenesis of the Kidney. In Developmental and Cell Biology Series. Vol. 19. P.W. Barlow, P.B. Green, and C.C. White, editors. Cambridge University Press, Cambridge, UK. 184 pp.
- Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D'Agati V, Pachnis V, Costantini F. Renal agenesis and hypodysplasia in ret-k−mutant mice result from defects in ureteric bud development. Development (Camb) 1996;122:1919–1929. doi: 10.1242/dev.122.6.1919. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschlesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Nagarajan L, Parada LF. c-ros: the vertebrate homolog of the sevenlesstyrosine kinase receptor is tightly regulated during organogenesis in mouse embryonic development. Development (Camb) 1992;115:11–20. doi: 10.1242/dev.115.1.11. [DOI] [PubMed] [Google Scholar]
- Treanor JJS, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F, et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- Trupp M, Arenas E, Fainzilber M, Nilsson A-S, Sieber B-A, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, Arumae U, Sariola H, Saarma M, Ibanez CF. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature. 1996;381:785–789. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- van Weering DHJ, Bos JL. Glial cell line-derived neurotrophic factor induces ret-mediated lamellipodia formation. J Biol Chem. 1997;272:249–254. doi: 10.1074/jbc.272.1.249. [DOI] [PubMed] [Google Scholar]
- van Puijenbroek AAFL, van Weering DHJ, van den Brink CE, Bos JL, van der Saag PT, de Laat SW, den Hertog J. Cell scattering of SK-N-MC neuroepithelioma cells in response to Ret and FGF receptor tyrosine kinase activation is correlated with sustained ERK2 activation. Oncogene. 1997;14:1147–1157. doi: 10.1038/sj.onc.1200911. [DOI] [PubMed] [Google Scholar]
- Vega QC, Worby CA, Lechner MS, Dixon JE, Dressler GR. Glial cell line-derived neurotrophic factor activates RET and promotes kidney morphogenesis. Proc Natl Acad Sci USA. 1996;93:10657–10661. doi: 10.1073/pnas.93.20.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf AS, Kolatsi-Joannou M, Hardman P, Andermarcher E, Moorby C, Fine LG, Jat PS, Noble MD, Gherardi E. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995;128:171–184. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]