Abstract
The ZAP-70 tyrosine kinase is essential for T cell activation by the T cell receptor. We show that ZAP-70 is also required for migration of T cells that is dependent on the integrin LFA-1. Invasion of TAM2D2 T cell hybridoma cells into fibroblast monolayers, which is LFA-1–dependent, was blocked by overexpression of dominant-negative ZAP-70 and by piceatannol but not by herbimycin A. The Syk inhibitor piceatannol blocks the Syk homologue ZAP-70, which is expressed by TAM2D2 cells, with the same dose dependence as the inhibition of invasion. Dominant-negative ZAP-70 completely inhibited the extensive metastasis formation of TAM2D2 cells to multiple organs upon i.v. injection into mice. Migration of TAM2D2 cells through filters coated with the LFA-1 ligand ICAM-1, induced by 1 ng/ml of the chemokine SDF-1, was blocked by anti–LFA-1 mAb and also abrogated by dominant-negative ZAP-70 and piceatannol. In contrast, migration induced by 100 ng/ml SDF-1 was independent of both LFA-1 and ZAP-70. LFA-1 cross-linking induced tyrosine phosphorylation, which was blocked by dominant-negative ZAP-70 and piceatannol. We conclude that LFA-1 engagement triggers ZAP-70 activity that is essential for LFA-1–dependent migration.
Keywords: integrin, activation, chemotaxis, SDF-1, metastasis
Activated T cells are highly motile and invade monolayers of hepatocytes and fibroblasts. T cell hybridomas, generated from activated T cells or from cytotoxic T cell clones, are also invasive and upon i.v. injection, these cells metastasize to many tissues (Roos et al., 1985; La Rivière et al., 1993). In contrast, the BW5147 lymphoma fusion partner used to generate these hybridomas is not invasive and does not metastasize at all. LFA-1–deficient mutants of the murine TAM2D2 T cell hybridoma had lost invasiveness and metastatic capacity (Roossien et al., 1989), indicating that the integrin LFA-1 (CD11a/CD18) is absolutely required. Furthermore, invasion was blocked by pertussis toxin (Roos and Van de Pavert, 1987) and transfection of the S1 catalytic subunit of the toxin strongly reduced metastasis (Driessens et al., 1996), albeit not completely. Recently, however, we did block metastasis completely with higher S1 levels obtained using retroviral transduction (see below), showing that Gi protein signals are absolutely required. The most likely explanation is that invasion depends on chemokines, present in monolayers and in tissues, that induce invasion upon binding to G protein–coupled receptors. Similarly, as proposed for various inflammation mediators (Lorant et al., 1991), an important effect of these chemokines may be the activation of leukocyte function-associated antigen-1 (LFA-1), since direct activation of LFA-1 with Mn2+ was sufficient to overcome the inhibition by pertussis toxin (La Rivière et al., 1994).
A chemokine that is potentially involved in this invasion is stromal cell-derived factor-1 (SDF-1).1 We found that its receptor, murine CXCR4, is expressed by TAM2D2 T cell hybridoma cells and that SDF-1 is present in fibroblast monolayers (Soede, R.D.M., unpublished observations). SDF-1 is expressed in many noninflamed tissues that are invaded by the T cell hybridomas, including the liver (Tashiro et al., 1993), and is a potent chemoattractant for T cells (Bleul et al., 1996). It is therefore a major candidate for being involved in both the in vitro invasion and in vivo metastasis of the T cell hybridomas, but this remains to be established. In the present study, we have used SDF-1 as a prototype of the chemokine(s) that may eventually turn out to be involved. In addition to the fibroblast monolayer assay we used, as a more simplified model for invasion, a chemotaxis assay based on a low concentration of SDF-1 (1 ng/ml) as chemoattractant and ICAM-1–coated filters. Similarly as in vitro invasion and in vivo metastasis, migration through the filters required LFA-1 and was blocked by pertussis toxin, whereas migration induced by high SDF-1 concentrations (100 ng/ml) was independent of LFA-1.
The zeta-associated protein-70 (ZAP-70) tyrosine kinase plays an essential role in the activation of T cells by the T cell receptor (TCR)–cluster of differentiation 3 (CD3) complex (Chan et al., 1994; Sloan-Lancaster et al., 1994; Madrenas et al., 1995), but has not been demonstrated to be involved in other processes. We show here that ZAP-70 is required for invasion and metastasis as well as LFA-1–dependent migration. In contrast, ZAP-70 is not involved in LFA-1–independent migration induced by a high concentration of SDF-1.
Materials and Methods
Cells and Invasion Assays
TAM2D2 and ESb cells and rat embryo fibroblasts (REFs) were grown as described previously (La Rivière et al., 1988). TAM2D2 cells were pretreated at 37°C with piceatannol (Boehringer GmbH, Mannheim, Germany) for 1 h or with herbimycin A (Calbiochem-Novabiochem, Inc., La Jolla, CA) for 20 h. Cells were washed twice with RPMI 1640 medium (GIBCO BRL, Paisley, Scotland, UK) and concentrated to 106 cells per ml of RPMI 1640. REFs in 24-well plates were washed twice with RPMI 1640 before addition of 5 × 105 TAM2D2 cells. After 1 h at 37°C, noninvaded cells were washed away and the monolayers were fixed with 2% paraformaldehyde. Invaded cells were counted using phase-contrast microscopy, as described previously (La Rivière et al., 1988). Invasion is expressed as the percentage of added cells that have invaded.
Generation and Transduction of DNA Constructs
The cDNAs encoding the wild-type or truncated human ZAP-70 (1–276) (Qian et al., 1996) were cloned into the retroviral vector pMFG-IRES-geo (geo = fusion protein of lacZ and neoR). An internal ribosome entry site (IRES) is located 3′ of this cDNA, followed by the cDNA encoding the neo–lacZ fusion protein, allowing the translation of both the ZAP-70 and geo protein from one bicistronic mRNA (Mountford et al., 1994). Thus, clones with high β-galactosidase activity can be selected from the neomycin-resistant transduced cells and this high lacZ activity correlates with high expression of ZAP-70 (Staal et al., 1996). The vector was transfected by CaPO4 2− precipitation into the virus-packaging cell line BOSC23 (Pear et al., 1993). TAM2D2 cells (2 × 105) were transduced with the virus by cocultivation or by incubation with the BOSC23 supernatant. After 1 d, TAM2D2 cells were transferred to fresh medium and after another day plated in 96-well plates (Costar Corp., Cambridge, MA) at 2,000–10,000 cells per well in 1 mg/ml G418 (GIBCO BRL). After 1–2 wk clones were stained for lacZ expression. If necessary, cells were subcloned or FACS-sorted™ to select clones with homogeneous high and stable lacZ expression. As controls, we generated cells expressing similarly high levels of the wild-type full-length ZAP-70 protein and cells expressing only the geo protein, the latter in the same vector, but without the IRES. In addition, we generated cells expressing the S1 catalytic subunit of pertussis toxin at higher levels than previously described for S1 transfectants. In these cells, Gi2 and Gi3 proteins were inactivated completely, determined similarly as previously described (Driessens et al., 1996).
In Vitro Kinase Assay
The pGEX-2T vector, containing the full-length coding sequence of the ZAP-70 substrate Sam68 was kindly provided by G. Bismuth (Centre Hospitalier, Pitié-Salpêtrière, Paris, France). Glutathione-S-transferase (GST)–Sam68 fusion proteins were isolated and the in vitro kinase assay was performed as described (Lang et al., 1997). In brief, fusion proteins were purified on glutathione–Sepharose beads (Pharmacia Diagnostics AB, Uppsala, Sweden), washed three times in a buffer (20 mM Tris-HCl, pH 7.5, 140 mM NaCl, 1 mM EDTA) containing 1% (vol/vol) NP-40, and once with phosphorylation buffer (50 mM Pipes, pH 6.8, 10 mM MnCl2, 10 mM MgCl2, 50 mM Na3VO4, and protease inhibitors). The beads were suspended in 30 μl phosphorylation buffer, supplemented with 10 μM cold ATP and 10 μCi [γ-32P]ATP, and the reaction was started by adding 400 ng purified recombinant ZAP-70, kindly provided by A. Isacchi (Department of Molecular Biology, Pharmacia & Upjohn, Milan, Italy). After 10 min at 20°C, the reaction was stopped by addition of Laemmli buffer and boiling. Proteins were separated by 10% SDS-PAGE, and the dried gel was subjected to autoradiography and quantified by phosphoimaging.
Immunoblotting
SDS-PAGE–separated cell lysates were blotted to nitrocellulose, which was then blocked with 3% BSA and 0.4% Tween-20. The membranes were incubated overnight with the mouse anti–human ZAP-70 mAb 2F3.2 (Qian et al., 1996) or rabbit anti–mouse ZAP-70 polyclonal antiserum 956 (Weil et al., 1995) at 4°C, followed by incubation with sheep anti–mouse or donkey anti–rabbit horseradish peroxidase-coupled Ig (Amersham Int., Little Chalfont, UK), respectively. Stained proteins were visualized by chemiluminescence (ECL kit; Amersham Int.).
Migration Assays
Transwells with pore diameters of either 8 or 5 μm (Costar Corp.) were coated overnight at 4°C with 1 μg/ml mouse soluble intercellular adhesion molecule-1 (ICAM-1) purified from 2706C7 cells as described (Welder et al., 1993), followed by blocking for 2 h at room temperature with 0.5% ovalbumin (Sigma Chemical Co., St. Louis, MO). TAM2D2 cells were washed twice with ice-cold RPMI 1640 and resuspended in ice-cold RPMI 1640 containing 0.1% ovalbumin at 0.66 × 106 cells/ml. Cells were pretreated with 10 μg/ml of the anti–LFA-1 α-chain antibody M17/4 (Sanchez-Madrid et al., 1983) or the anti-α6 antibody GoH3 (Sonnenberg et al., 1988) for 30 min, or with 50 μg/ml piceatannol, as described above. The lower chamber was filled with 250 μl RPMI 1640 containing 0.1% ovalbumin and the indicated concentration of SDF-1α (Pepro Tech, Rocky Hill, NJ), the Transwell placed on top, and 150 μl medium with 105 cells inserted into the upper chamber. After 2 h at 37°C, the cells present in the lower chamber were counted. The data presented are the percentages of added cells that have migrated in response to the indicated concentration of SDF-1α through the ICAM-1–coated filter and have been collected from the lower chamber.
Migration of T Cells
The mouse cytotoxic T cell clone 10.1.1, specific for the ovalbumin-derived peptide SIINFEKL (Van Bleek, G., unpublished results), was restimulated with EG7OVA cells (chicken ovalbumin-transfected EL4 cells; Moore et al., 1988) and interleukin 2. The cells were harvested 10 d later and used in migration assays as described above.
Tyrosine Phosphorylation Induced by LFA-1 Cross-linking
Cells were incubated for 20 min on ice with F(ab′) fragments of the M17/4 mAb against the LFA-1 α-chain, at 2 × 106 cells per ml of Hank's balanced salt solution (HBSS; GIBCO BRL), pH 7.0, supplemented with 20 mM Hepes, 0.35 g/liter NaHCO3, 1 mM CaCl2 and 1 mM MgCl2. LFA-1 molecules were then cross-linked by addition of rabbit anti–rat Ig antibodies (dilution 1:200; Nordic Immunology, Tilburg, The Netherlands), and incubation for 30 min at 37°C. Cross-linking was stopped by adding ice-cold stopping buffer (0.4 mM EDTA, 10 mM NaF, and 0.1 mM Na3VO4 in HBSS, pH 7.4). Cells were pelleted by centrifugation and lysed immediately in ice-cold lysis buffer (100 mM NaCl, 2 mM CaCl2, 1% Nonidet P-40, 10% glycerol, 10 mg/ml aprotinin, 1 mg/ml leupeptin, 50 mM pefablock, 10 mM NaF and 0.1 mM Na3VO4 in 25 mM Tris, pH 7.5). After 30 min on ice, insoluble material was removed by centrifugation. Immunoblotting was performed as described above, except that membranes were incubated for 1 h with the anti-phosphotyrosine mAb PY20 (Transduction Laboratory, Lexington, KY). The same membrane was reprobed with rabbit anti–mouse ZAP-70 Ab 956.
Results
Role of Syk Family Kinases in Invasion
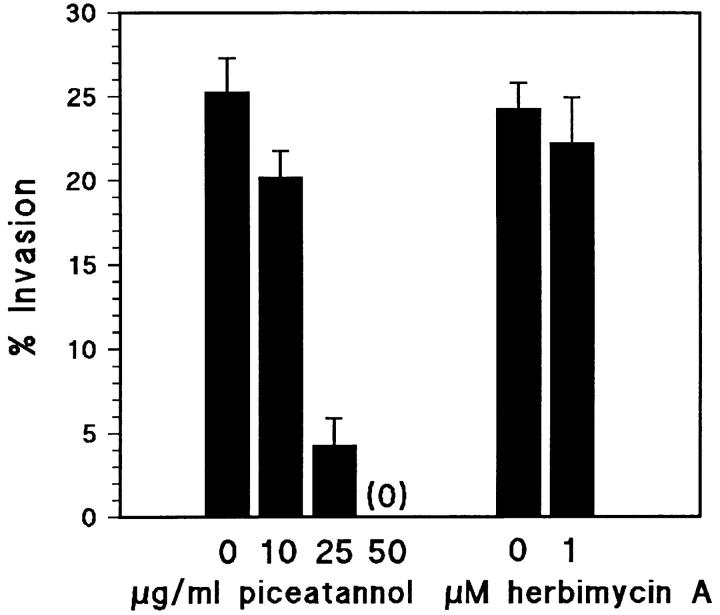
We found that piceatannol, a specific inhibitor of the Syk tyrosine kinase, completely blocked the invasion of fibroblast monolayers by the TAM2D2 mouse T cell hybridoma (Fig. 1). The dose dependence was the same as reported for inhibition of Syk activity, with a complete block at 50 μg/ml, a concentration at which Src family kinases are not affected (Oliver et al., 1994; Keely and Parise, 1996). In contrast, 1 μM herbimycin A which blocks Src-like kinases (Ericsson and The, 1995) did not inhibit invasion, whereas adhesion of the cells to ICAM-1 induced by activation of G proteins with AlF4 − (Driessens et al., 1997) was blocked by herbimycin A but not by piceatannol (data not shown). Reverse transcription-PCR analysis showed that ZAP-70 was expressed in TAM2D2 cells, whereas Syk was not. As a positive control, we used ESb mouse lymphoma cells which expressed Syk but not ZAP-70 (data not shown). This suggested that piceatannol blocked the activity of ZAP-70. Indeed, using purified recombinant ZAP-70 and a GST fusion protein of the ZAP70 substrate Sam68 (Lang et al., 1997) in an in vitro kinase assay, we observed that ZAP-70 activity was inhibited with the same dose dependence as reported for Syk (Fig. 2) and as found for inhibition of invasion (Fig. 1).
Figure 1.
The Syk/ZAP-70 tyrosine kinase inhibitor piceatannol blocks invasion of TAM2D2 T cell hybridoma cells into REF monolayers. Cells located under the monolayer were counted after 1 h of incubation and the percentage of added cells that had invaded was calculated. Data are averages ± SE from three experiments performed in duplicate.
Figure 2.
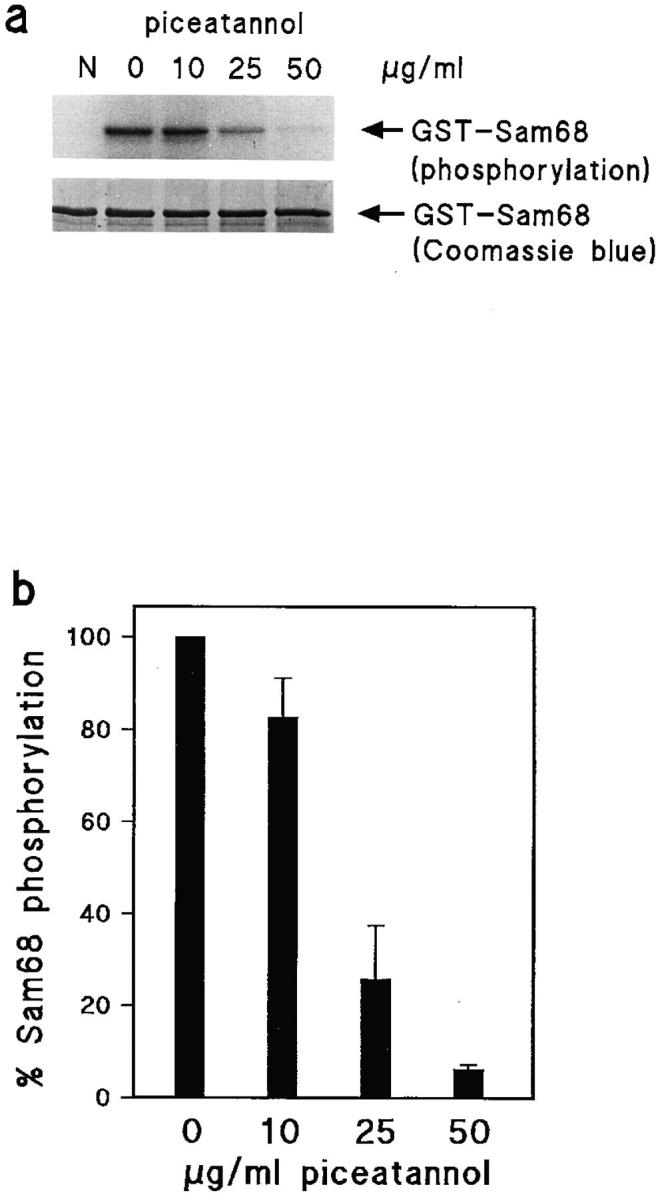
Inhibition of ZAP-70 kinase activity by piceatannol. (a) Phosphorylation of the ZAP-70 substrate Sam68 in the presence of increasing concentrations of piceatannol. N, negative control (no ZAP-70 added). Equal loading of the gel is indicated by Coomassie blue staining of Sam68. (b) Quantification of kinase activity by phosphoimaging. Data shown are percentages of Sam68 phosphorylation in the absence of piceatannol and are averages of two independent experiments.
Inhibition of Invasion and Metastasis by Dominant-negative ZAP-70
To further study the possible role of ZAP-70 in invasion, we generated TAM2D2 cells expressing high levels of a truncated ZAP-70 protein shown to inhibit T cell receptor signaling in a dominant-negative fashion (Qian et al., 1996). This was achieved by retroviral transduction of a bicistronic vector that contained two cDNAs separated by an IRES (Mountford et al., 1994). The first encodes a truncated ZAP-70 protein and the second a lacZ–neomycin (geo) fusion protein (Staal et al., 1996). Since expression levels of the two cDNAs correlate, it is possible to select clones with homogeneous high expression levels of the truncated ZAP-70 protein, based on high lacZ activity.
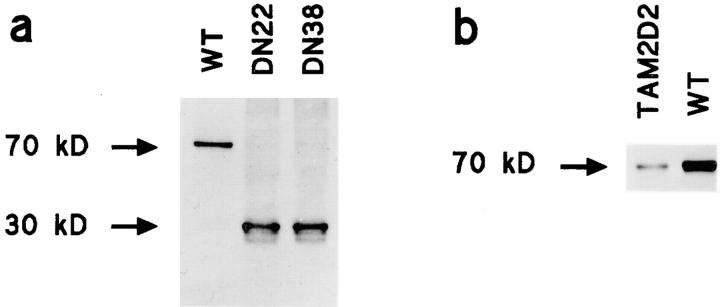
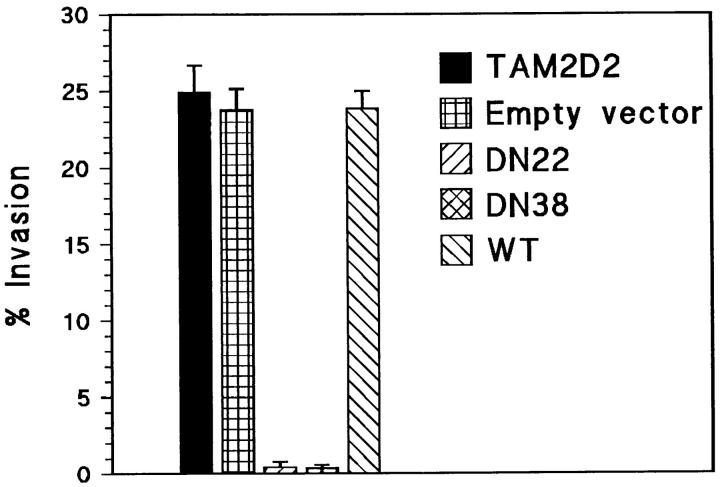
Many independent clones expressing this dominant-negative ZAP-70 were obtained, which exhibited a reduction in invasive capacity that correlated with lacZ expression level. Six independent clones with high lacZ expression had completely lost invasiveness. Two of these, termed DN22 and DN38, were studied in more detail. As controls, we used clones transduced with the empty vector or overexpressing wild-type full-length ZAP-70 (WT). The truncated 30-kD and the wild-type 70-kD human ZAP-70 proteins were expressed at similar levels in, respectively, the DN22 and DN38 cells and the WT clone used for comparison (Fig. 3 a). This was substantially higher than the level of endogenous mouse ZAP-70 (Fig. 3 b), at least 10-fold as indicated by densitometry. The DN22 and DN38 cells, expressing the dominant-negative ZAP-70, did not invade the fibroblast monolayers at all (Fig. 4), whereas control transfectants that overexpressed wild-type ZAP-70 (WT) or only the empty vector were as invasive as the parental TAM2D2 cells. The overexpressed ZAP-70 proteins did not affect LFA-1 levels, which were the same on all cells.
Figure 3.
Expression levels of transduced overexpressed dominant-negative and wild-type ZAP-70. (a) Levels of overexpressed wild-type and dominant-negative human ZAP-70 in TAM2D2 clones WT, DN22, and DN38, detected by the mouse anti–human ZAP-70 mAb 2F3.2. (b) Level of overexpressed human ZAP-70 in TAM2D2 clone WT compared with level of endogenous ZAP-70 in TAM2D2 cells, detected by the rabbit anti–mouse ZAP-70 polyclonal Ab 956.
Figure 4.
Invasion is blocked by dominant-negative ZAP-70 (1– 276). Shown are the percentages of cells that have invaded fibroblast monolayers after 1 h. Parental TAM2D2 cells, cells transduced with empty vector, and WT cells overexpressing full-length intact ZAP-70 invade rapidly and extensively, but the DN22 and DN38 cells overexpressing dominant-negative ZAP-70 (1–276) do not invade at all. Data are averages ± SE of three experiments performed in duplicate.
TAM2D2 cells (5 × 105) were injected into the tail vein of nude mice. Similarly as previously described (Roos et al., 1985; La Rivière et al., 1988, 1993), these cells invaded multiple tissues, predominantly the liver, spleen, kidneys, and ovaries, and all mice (10 out of 10) died within 4 wk as a consequence of the massive proliferation of the cells within these tissues. The same was true for 10 out of 10 mice injected with the empty vector transfectant, which had similar lacZ activity as the DN22 and DN38 cells. Mice were also injected with the dominant-negative ZAP-70–expressing cells, 10 mice with DN22 and 10 with DN38 cells, and the animals were killed after 4 wk. In none of these 20 mice did we observe any metastasis, as shown for liver and spleen metastasis in Fig. 5, a and b. In a second experiment, we assessed the survival of the mice. As shown in Fig. 5 c, all mice injected with TAM2D2 cells and the empty vector transfectants were moribund within ∼4 wk with extensive metastasis. In contrast, mice injected with the DN22 and DN38 cells survived for 100 d. They were then killed and no macroscopic metastasis was observed. The same was true for cells transduced with the S1 catalytic subunit of pertussis toxin. Previously, we described the greatly reduced metastatic capacity of TAM2D2 cells transfected with a plasmid S1 expression vector, with low S1 levels and incomplete (95%) Gi protein inactivation (Driessens et al., 1996). The retrovirally transduced cells we now generated had much higher S1 levels and completely inactivated Gi proteins, assessed similarly as described previously (Driessens et al., 1996), and did not metastasize at all. The residual metastasis previously observed was therefore clearly due to the 5% remaining Gi protein activity. After intraperitoneal injection, DN22, DN38, and S1-transduced cells proliferated in the abdominal cavity with a doubling time similar to TAM2D2 cells (data not shown), showing that the lack of metastasis was not due to rejection or inability to proliferate in vivo.
Figure 5.
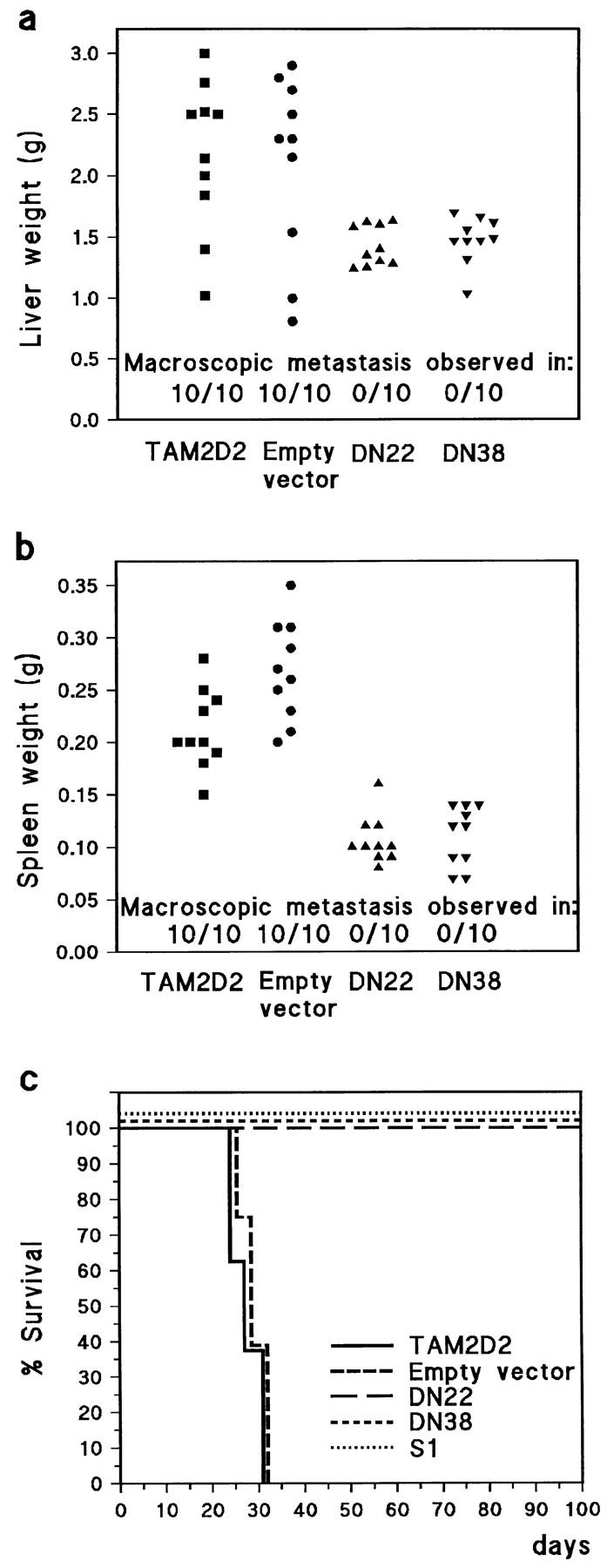
Metastasis is blocked by dominant-negative ZAP-70 (1–276) and by pertussis toxin S1. (a) Extent of metastasis to liver as measured by weight, ∼4 wk after tail vein injection of 5 × 105 cells into nude mice. Also the presence of macroscopically visible metastatic foci is indicated. Mice were killed when moribund or after 4 wk. Each group consisted of 10 mice. (b) Spleen weights in the same experiment. (c) Survival curve of nude mice tail vein-injected with 5 × 105 cells in a separate experiment. Mice were killed when moribund or after 100 d. Each group consisted of eight mice. All mice injected with cells expressing dominant-negative ZAP-70 (DN22 and DN38) or pertussis toxin S1 survived, and no metastases were found after 100 d. All cells proliferated at a similar rate when injected intraperitoneally (data not shown).
SDF-1–induced Migration: Role of LFA-1
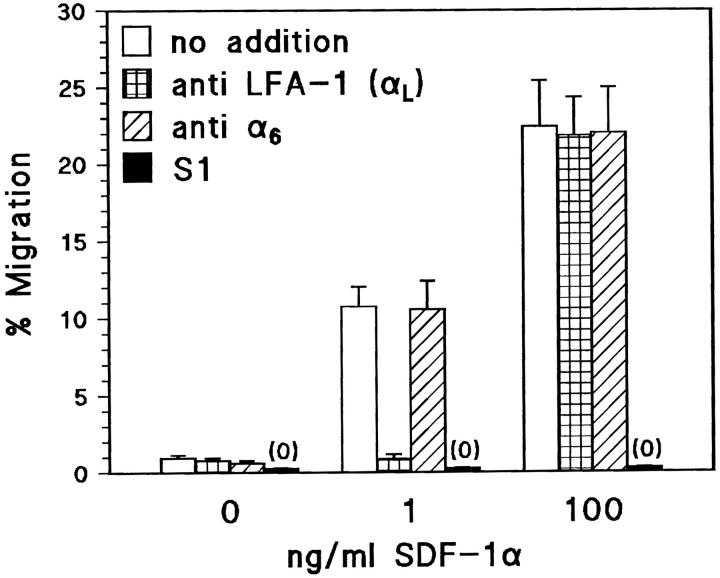
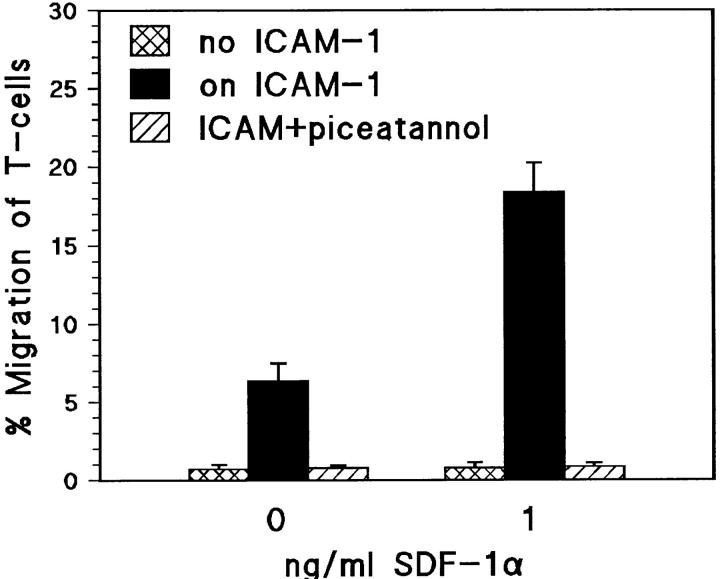
We developed a chemotaxis assay as a simplified model for invasion using SDF-1 as chemoattractant and filters coated with the LFA-1 ligand ICAM-1. SDF-1 is a major candidate for being the chemokine that activates LFA-1 during invasion (refer to Introduction). The difference with previous reports describing the chemotactic activity of SDF-1 (Bleul et al., 1996) is that we used a thousand-fold lower concentration of SDF-1α:1 ng/ml. At this concentration, SDF-1 induced rapid migration of TAM2D2 cells through the filters, but only if these were coated with ICAM-1. After 2 h, ∼10% of the cells could be collected from the lower compartment of the Transwells (Fig. 6). This migration was completely blocked by an mAb against the α-subunit of LFA-1, but not by a control mAb against the α6 subunit of the α6β1 integrin, which is also expressed by these cells. In striking contrast, migration induced by a high concentration of SDF-1 (100 ng/ml) was completely independent of LFA-1, since it was not affected at all by the anti–LFA-1 mAb (Fig. 6). In fact, the extent of migration was similar through filters that were not coated with ICAM-1 (data not shown). Most migration assays were performed with filters having 8-μm pores, but the extent of migration was quantitatively similar with 5-μm pores (24% migration in 2 h at 100 ng/ml SDF-1, LFA-1–independent, and 12% at 1 ng/ml, LFA-1–dependent). This excludes the trivial possibility that SF-1–induced shape changes would allow the cells to fall through the 8-μm pores.
Figure 6.
SDF-1α–induced migration through ICAM-1–coated filters is LFA-1–dependent at a low, but not at a high SDF-1 concentration. Data are the percentages of cells that have migrated in 2 h through an ICAM-1–coated filter to the lower chamber of a Transwell, containing the indicated SDF-1 concentration. Shown is the effect of the M17/4 mAb against LFA-1, with the GoH3 mAb against the α6 integrin subunit as control. M17/4 blocks completely at 1 ng/ml SDF-1, but not at all at 100 ng/ml. At both concentrations, migration depends on pertussis toxin-sensitive G proteins, since TAM2D2 cells expressing high levels of the S1 catalytic subunit of pertussis toxin do not migrate at all. Data are averages ± SE of two experiments performed in duplicate.
The Gi protein signals, triggered by the binding of SDF-1 to its receptor, are always required. This was demonstrated with the cells transduced with the S1 catalytic subunit of pertussis toxin, with completely inactivated Gi proteins (see above). These cells did not migrate at all at either the high or low concentration of SDF-1 (Fig. 6).
LFA-1–dependent Migration Requires ZAP-70
Similarly as for invasion and metastasis, ZAP-70 was observed to be absolutely required for the LFA-1–dependent migration induced by 1 ng/ml SDF-1. The dominant-negative ZAP-70–expressing clones DN22 and DN38 had completely lost the ability to migrate at this low SDF-1 concentration whereas WT cells, overexpressing the full-length intact ZAP-70 protein, migrated normally (Fig. 7 a). Furthermore, piceatannol completely blocked migration of the parental TAM2D2 cells (Fig. 7 b). In striking contrast, neither the dominant-negative ZAP-70 nor piceatannol influenced the LFA-1–independent migration at 100 ng/ml SDF-1.
Figure 7.
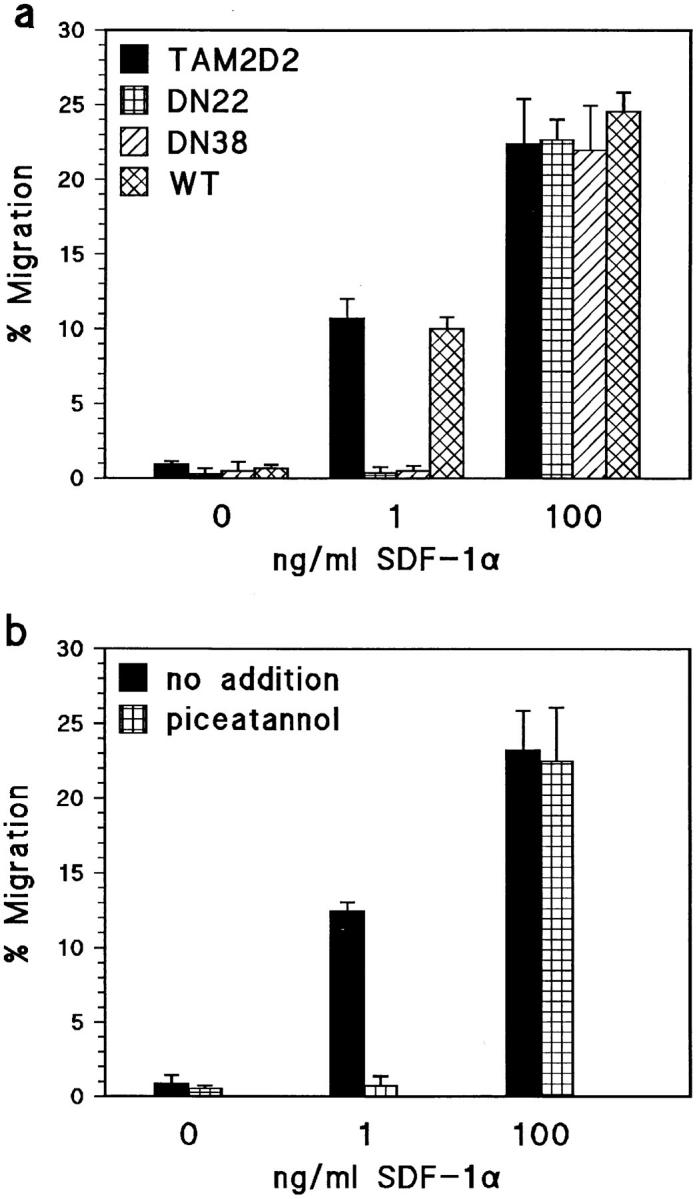
SDF-1a–induced migration through ICAM-1–coated filters requires ZAP-70 at a low, but not at a high SDF-1 concentration. (a) Migration of the DN22 and DN38 cells expressing dominant-negative ZAP-70, parental TAM2D2 cells, and cells overexpressing wild-type ZAP-70 (WT). In contrast to TAM2D2 and WT cells, DN22 and DN38 cells do not migrate at all at 1 ng/ml SDF-1. At 100 ng/ml SDF-1, all cells migrate to the same extent. (b) Migration of TAM2D2 cells is blocked completely by piceatannol. Data are averages ± SE of two experiments performed in duplicate.
Role of ZAP-70 in Migration of Normal T Cells
Since the T cell hybridomas derived their invasiveness from the normal T cell fusion partner, the above results suggested that ZAP-70 is not only involved in T-lymphoma metastasis but also in the migration of normal T cells. Unfortunately, this can not be tested with T cells from ZAP-70 knock-out mice because development of mature T cells is blocked in these mice (Negishi et al., 1995). However, the notion is supported by the effect of piceatannol on migration of a cytotoxic T cell clone (Fig. 8). Even in the absence of chemoattractant, these cells migrated spontaneously through ICAM-1–coated but not uncoated filters, but this LFA-1–dependent migration was threefold increased by 1 ng/ml SDF-1. Both the spontaneous and the SDF-1–enhanced migration were completely blocked by piceatannol.
Figure 8.
Piceatannol blocks LFA-1–dependent migration of a cytotoxic T cell clone. Shown are the percentages of cells that have migrated to the lower well of a Transwell chamber, containing either no chemoattractant or 1 ng/ml SDF-1. The filters were either uncoated or ICAM-1–coated, and in the latter case the assay was performed in the presence or absence of 50 μg/ml piceatannol. Data are averages ± SE of three experiments performed in duplicate.
ZAP-70 Is Required for LFA-1–induced Tyrosine Phosphorylation
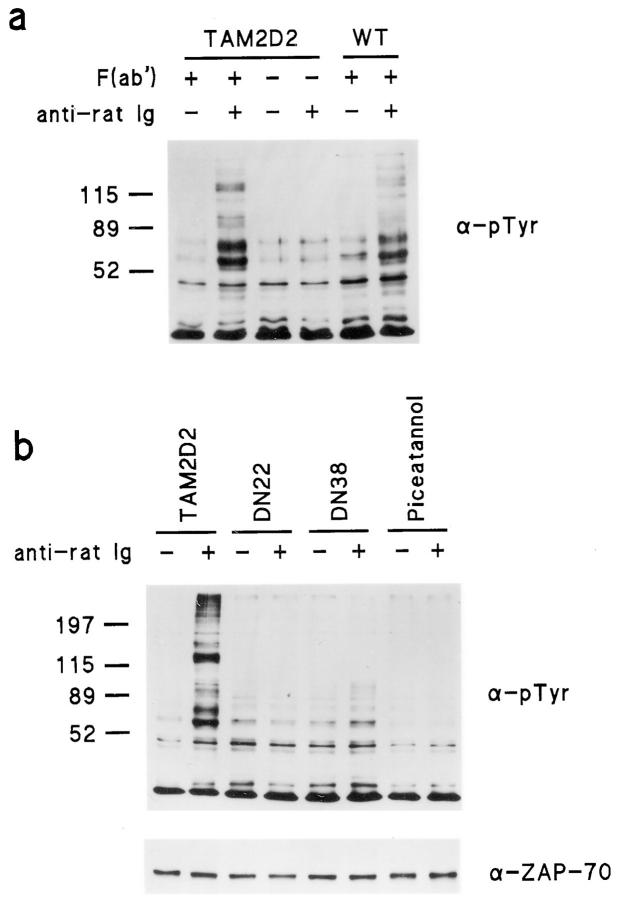
ZAP-70 activation by LFA-1 probably occurs locally and transiently, e.g., upon initial contact of cell extensions with ICAM-1, on cells or on the ICAM-1–coated substrate, and can not be demonstrated in cells that have invaded or migrated. However, cross-linking of integrins with blocking antibodies has been shown to elicit similar responses as ligand binding (Miyamoto et al., 1995). Thus, we have mimicked LFA-1 engagement by cross-linking of F(ab) fragments of the M17/4 blocking anti–LFA-1 mAb and anti-Ig polyclonal antibodies. This induced extensive tyrosine phosphorylation of multiple proteins in both the parental TAM2D2 cells and in cells overexpressing the intact wild-type ZAP-70, whereas exposure to second antibody alone had no such effect (Fig. 9 a). The LFA-1–induced phosphorylation did not occur in the DN22 and DN38 cells that overexpress the dominant-negative ZAP-70, and in TAM2D2 cells it was blocked by piceatannol (Fig. 9 b).
Figure 9.
LFA-1–induced tyrosine phosphorylation in TAM2D2 cells is due to ZAP-70 activation. LFA-1 molecules were cross-linked using F(ab′) fragments of the rat mAb M17/4 against LFA-1 and anti-rat Ig antibodies and then immunoblotting was performed on cell lysates using the PY20 mAb against phosphotyrosine. (a) LFA-1 cross-linking induces tyrosine phosphorylation in TAM2D2 cells and WT cells overexpressing wild-type ZAP-70, whereas anti-rat Ig antibodies alone have no such effect in TAM2D2 cells. (b) Tyrosine phosphorylation occurs in TAM2D2 cells, but not in the DN22 and DN38 cells overexpressing dominant-negative ZAP-70 and in TAM2D2 cells treated with piceatannol.
Discussion
We show here that signals triggered by LFA-1 engagement are essential for T cell invasion into fibroblast monolayers as well as metastasis of T cell hybridoma cells in vivo, and require the activity of the ZAP-70 tyrosine kinase. This is also true for migration induced by low, but not by high concentrations of SDF-1. At high SDF-1 concentrations, migration was completely independent of LFA-1 and the presence of the LFA-1 ligand ICAM-1 was irrelevant. This migration was not affected by overexpressed dominant-negative ZAP-70 and not by piceatannol, showing that the observed effects on migration at low SDF-1 concentrations were not due to acute toxicity or a general effect on the migration machinery. Migration induced by high SDF-1 levels is dependent on G protein signaling since it was completely blocked in cells expressing the S1 catalytic subunit of pertussis toxin. Preliminary results with inhibitors further indicate a role for phospholipase C but not phosphatidylinositol-3-kinase (data not shown).
The signaling requirements are quite different at low SDF-1 concentrations. Migration was completely dependent on LFA-1, and was blocked by dominant-negative ZAP-70 and piceatannol. Furthermore, preliminary results indicate that it does require phosphatidylinositol-3-kinase. Since adhesion to ICAM-1 is not necessary for migration induced by high SDF-1 concentrations, it seems likely that the major role of LFA-1 is not to provide substrate attachment, but rather to transduce signals upon ICAM-1 engagement. Costimulation by these signals is apparently required when the number of occupied SDF-1 receptors is insufficient to trigger migration. However, the chemokine signals remain essential for the T cell hybridoma cells used, since migration was still completely blocked by pertussis toxin S1 and did not occur in the absence of SDF-1. Both LFA-1 and Gi protein function are essential for in vivo metastasis and in vitro invasion of T cell hybridoma cells as we have demonstrated with LFA-1–deficient mutants (Roossien et al., 1989) and cells expressing pertussis toxin S1 (Driessens et al., 1996). We show here that ZAP-70 is essential as well. These signal requirements are the same as for migration induced by low SDF-1 levels through ICAM-1–coated filters, which therefore appears to be a suitable model for essential steps in T cell invasion and T lymphoma metastasis, and more appropriate than migration induced by high chemokine concentrations. For the T cell clone used in this study (refer to Fig. 8), the requirement for chemokine signaling was less stringent at these ICAM-1 densities, possibly due to an autocrine factor, but migration was greatly enhanced by SDF-1. However, both the spontaneous and chemokine-enhanced LFA-1–dependent migration were blocked by piceatannol and therefore likely to be dependent on ZAP-70 activity.
The involvement of ZAP-70 in integrin signaling and motility is a novel finding but is consistent with recent results on the ZAP-70 homologue Syk, which is one of the proteins that is tyrosine-phosphorylated upon cross-linking or activation of β1, β2, and β3 integrins in hematopoietic cells (Clark et al., 1994; Lin et al., 1995; Gotoh et al., 1997; Yan et al., 1997). However, activation of Syk by the integrin αIIbβ3 was recently shown to involve Src, and this activation was not blocked by a truncated Syk containing the SH2 domains (Gao et al., 1997). In contrast, the ZAP-70 activation by LFA-1 that we observed, does not involve Src-like kinases (see below). Furthermore, we did see inhibition by a truncated ZAP-70, although this is somewhat shorter and, unlike the truncated Syk, does not contain the interdomain B. This truncated ZAP-70 (1–276), which inhibits T cell receptor signaling in a dominant-negative fashion (Qian et al., 1996), consists mainly of the two NH2-terminal SH2 domains and the interdomain A. Although interactions of SH2 domains are quite specific, it is conceivable that these SH2 domains interfere with functions of other proteins. However, this can be excluded since the SH2 domains in the wild-type intact ZAP-70, at similar expression levels, did not inhibit migration, invasion, and metastasis. Therefore, we conclude that the observed effects are due to specific interference with ZAP-70 function. The inhibition by ZAP-70 (1–276) thus shows that the tyrosine phosphorylation triggered by LFA-1 cross-linking is due to induced ZAP-70 activity. This phosphorylation was also blocked by 50 μg/ml piceatannol, previously reported to inhibit the activity of the ZAP-70 homologue Syk but not of the Src-like kinase Lyn (Oliver et al., 1994). Piceatannol inhibited ZAP-70 activity and invasion with the same dose dependence as reported for Syk (refer to Figs. 1 and 2). In contrast, herbimycin A had little effect on invasion at a concentration that inhibits the Src-like kinase Lck (Ericsson and The, 1995). This is in line with a report by Sugie et al. (1995) that the tyrosine kinase downstream of LFA-1 is not Src-like, and also supported by our preliminary results with cells overexpressing high levels of dominant-negative Lck, which had no effect on invasiveness. Conversely, AlF4 −-induced adhesion to ICAM-1 was blocked by herbimycin A, but not by piceatannol. This indicates that ZAP-70 has no role in LFA-1 activation induced by AlF4 −-activated G proteins and suggests involvement of Lck or another Src-like kinase, which are not required for invasion. However, AlF4 − stimulates all heterotrimeric G proteins and the effect on adhesion may be due to activation of pertussis toxin-insensitive Gq proteins that so far have not been found to play a role in invasion.
The effect of the truncated ZAP-70 suggests involvement of a protein containing an immunoreceptor family tyrosine-based activation motif (ITAM) sequence. In T cells, ITAM sequences are present in the TCR-ζ, -γ, -δ, and the CD3ε chains. However, the TCR–CD3 complex is not expressed by the TAM2D2 T cell hybridoma cells. Furthermore, these sequences require phosphorylation by Src-like kinases such as Lck (Iwashima et al., 1994), which do not appear to be involved in LFA-1 signaling in invasion (see above). Recently, it was shown that TCR-induced activation of phospholipase C-γ1 and calcium mobilization, which depend on ZAP-70, do not require both ITAM tyrosines. The NH2-terminal tyrosine is sufficient for ZAP-70 activation, although not for stable binding of ZAP-70 to the TCR (Sunder-Plassmann et al., 1997). It is thus conceivable that interaction of only one SH2 domain with a non-ITAM sequence causes ZAP-70 activation during LFA-1–dependent migration. In fact, transfected ZAP-70 translocates to the plasma membrane upon pervanadate treatment of COS7 cells (Sloan-Lancaster et al., 1997). COS7 cells do not contain known ITAM-containing receptors, indicating that ZAP-70 can interact with other tyrosine-phosphorylated molecules. Alternatively, the interdomain A between the SH2 domains may be involved. This domain has a coiled-coil structure and may mediate protein–protein interactions (Hatada et al., 1995). Future studies will have to discriminate between these possibilities.
We have not detected enhanced ZAP-70–induced phosphorylation in cells that have migrated through the filters, probably because ZAP-70 is activated only locally and transiently, e.g., upon contact of a cell extension with ICAM-1 on the substrate. However, signaling triggered by integrin engagement can be mimicked by antibody-mediated cross-linking (Miyamoto et al., 1995). Cross-linking of LFA-1 caused ZAP-70 activation, suggesting that ZAP-70 acts downstream of LFA-1. Gi protein-induced mitogen-activated protein kinase activation involves Syk (Wan et al., 1996) and activation of LFA-1 by SDF-1 may thus involve ZAP-70, but so far we have no indications for this possibility, and clearly the Gi protein-induced signals that trigger migration at high SDF-1 concentrations do not involve ZAP-70. Recently, we found that LFA-1 cross-linking by subsaturating concentrations of an anti-LFA1 mAb induced aggregation of TAM2D2 T cell hybridoma cells. This aggregation was mediated by the unoccupied LFA-1 molecules and thus apparently involves LFA-1–induced LFA-1 activation. In preliminary experiments, aggregation was blocked by dominant-negative ZAP-70 and by piceatannol (Soede, R.D.M., unpublished observations). If confirmed, this might explain why ZAP-70 is only involved in migration that depends on LFA-1. Occupation of a few SDF-1 receptors may lead to local activation of LFA-1 molecules which, upon interaction with ICAM-1, activate ZAP-70. This then leads to further activation of LFA-1 molecules that can repeat the process and propagate the signal.
Immunoprecipitated ZAP-70 was found to be tyrosine-phosphorylated in untreated TAM2D2 cells and LFA-1 cross-linking triggered little if any increase in this phosphorylation (data not shown). This differs from the ZAP-70 phosphorylation induced upon T cell receptor activation. In that case, however, the positive regulatory tyrosine is phosphorylated by Src-like kinases, which have no role here (see above), whereas the other phosphorylated tyrosines have a negative regulatory function (Kong et al., 1996). The Src-like kinase-mediated phosphorylation seen in T cell receptor signals quite probably reflects a stronger and more persistent activation of ZAP-70, as compared with a more limited and transient activation after LFA-1 engagement, required for LFA-1–dependent cell migration.
One of the substrates of Syk-like kinases is the adaptor protein Cbl (Fitzer-Attas et al., 1997) which binds to phosphorylated ZAP-70 (Fournel et al., 1996; Lupher et al., 1996), and the phosphorylated Cbl binds to several other ZAP-70 substrates. These include phospholipase C-γ (Donovan et al., 1994) and the p85 subunit of phosphatidylinositol-3-kinase (Hartley et al., 1995), which have been implicated in cell motility (Chen et al., 1994; Kundra et al., 1994; Wennström et al., 1994). Indeed, phospholipase C-γ1 is activated upon LFA-1 engagement (Kanner et al., 1993). T cell hybridoma invasion is abrogated by inhibitors of these enzymes (Soede, R.D.M., unpublished observations). Cbl also binds to the ZAP-70 substrate Vav (Smit et al., 1996; Wu et al., 1997), which is phosphorylated upon LFA-1 engagement (Zheng et al., 1996). When tyrosine-phosphorylated, Vav is a GDP/GTP exchanger and consequently an activator of Rho-like GTPases, in particular of Rac (Olson et al., 1996; Crespo et al., 1997). Since constitutively active Rac can induce invasiveness in noninvasive T lymphoma cells (Michiels et al., 1995), the activation of Rac, induced by ZAP-70 and Vav, is also likely to play a role in T cell invasiveness. Thus, ZAP-70 is responsible for both the assembly and the activation of a complex of proteins that are required for motility, perhaps in part for the sequential activation of LFA-1 molecules. The contribution of each of these proteins is under investigation.
We conclude that ZAP-70, in addition to being crucial for T cell activation, is also essential for LFA-1 activity during T cell migration and invasion. This is likely true not only for the tumorigenic T cell hybridomas studied, but also for normal T cells, given the effect of piceatannol on LFA-1–dependent migration of a T cell clone, as described here. T cell migration into tissues in transplantation reactions and autoimmune disease can be completely blocked by anti–LFA-1 and anti–ICAM-1 mAbs (Isobe et al., 1992; Hasegawa et al., 1994). Our results suggest that ZAP-70– specific antagonists will suppress such migration as well.
Acknowledgments
We thank A. Weiss (University of California, San Francisco, CA) for the mouse anti–human ZAP-70 2F3.2 mAb, and the pBJ1-ZAP-70 (1–276) and pBJ1-ZAP-70 wt vectors; A. Veillette (McGill University, Montreal, Canada) for the rabbit anti–mouse ZAP-70 Ab 956; G. Bismuth for GST– Sam68, A. Isacchi for recombinant ZAP-70, G. van Bleek for the T cell clone 10.1.1, and J. Cheung (University of Amsterdam, Amsterdam, The Netherlands) for her contribution to this project.
Abbreviations used in this paper
- CD3
cluster of differentiation 3
- GST
glutathione-S-transferase
- ICAM-1
intercellular adhesion molecule-1
- IRES
internal ribosome entry site
- LFA
leukocyte function-associated antigen-1
- SDF-1
stromal cell-derived factor-1
- TCR
T cell receptor
- WT
wild-type
- ZAP-70
zeta-associated protein-70
Footnotes
This research was supported by a grant from the Dutch Cancer Society (NKI 95-969).
Address all correspondence to E. Roos, Division of Cell Biology, The Netherlands Cancer Institute, 121 Plesmanlaan, 1066 CX Amsterdam, The Netherlands. Tel.: (31) 20-5121931. Fax: (31) 20-5121944. E-mail: eroos@nki.nl
References
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AC, Desai DM, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immun. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- Chen P, Xie H, Sekar MC, Gupta K, Wells A. Epidermal growth factor receptor-mediated cell motility: phospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J Cell Biol. 1994;127:847–857. doi: 10.1083/jcb.127.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Shattil SJ, Ginsberg MH, Bolen J, Brugge JS. Regulation of the protein tyrosine kinase pp72sykby platelet agonists and the integrin αIIbβ3. J Biol Chem. 1994;269:28859–28864. [PubMed] [Google Scholar]
- Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the Vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- Donovan JA, Wange RL, Langdon WY, Samelson LE. The protein product of the c-cbl protooncogene is the 120 kDa tyrosine-phosphorylated protein in Jurkat cells activated via the T cell antigen receptor. J Biol Chem. 1994;269:22921–22924. [PubMed] [Google Scholar]
- Driessens MHE, Van Rijthoven EAM, La Rivière G, Roos E. Expression of pertussis toxin adenosine diphosphate-ribosyltransferase in a T cell hybridoma reduces metastatic capacity. Blood. 1996;88:3116–3123. [PubMed] [Google Scholar]
- Driessens MHE, Van Hulten PEM, Van Rijthoven EAM, Soede RDM, Roos E. Activation of G proteins with AlF4 −induces LFA-1-mediated adhesion of T cell hybridoma cells to ICAM-1, by signal pathways that differ from phorbol ester- and manganese-induced adhesion. Exp Cell Res. 1997;231:242–250. doi: 10.1006/excr.1996.3463. [DOI] [PubMed] [Google Scholar]
- Ericsson PE, The HS. The protein tyrosine kinase p56lckregulates TCR expression and T cell selection. Intern Immunol. 1995;7:617–624. doi: 10.1093/intimm/7.4.617. [DOI] [PubMed] [Google Scholar]
- Fitzer-Attas CJ, Schindler DG, Waks T, Eshhar Z. Direct T cell activation by chimeric single chain Fv-Syk promotes Syk-Cbl association and Cbl phosphorylation. J Biol Chem. 1997;272:8551–8557. doi: 10.1074/jbc.272.13.8551. [DOI] [PubMed] [Google Scholar]
- Fournel M, Davidson D, Weil R, Veillette A. Association of tyrosine protein kinase ZAP-70 with the proto-oncogene product p120c-cblin T lymphocytes. J Exp Med. 1996;183:301–306. doi: 10.1084/jem.183.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Zoller KE, Ginsberg MH, Shattil SJ. Regulation of the pp72sykprotein tyrosine kinase by platelet integrin αIIbβ3. EMBO (Eur Mol Biol Organ) J. 1997;16:6414–6425. doi: 10.1093/emboj/16.21.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh A, Takahira H, Geahlen RL, Broxmeyer HE. Cross-linking of integrins induces tyrosine phosphorylation of the proto-oncogene product Vav and the protein tyrosine kinase Syk in human factor-dependent myeloid cells. Cell Growth Differ. 1997;8:721–729. [PubMed] [Google Scholar]
- Hartley D, Meisner H, Corvera S. Specific association of the β isoform of the p85 subunit of phosphatidylinositol-3-kinase with the proto-oncogene c-Cbl. J Biol Chem. 1995;270:18260–18263. doi: 10.1074/jbc.270.31.18260. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Yokono K, Taki T, Amano K, Tominaga Y, Yoneda R, Yagi N, Maeda S, Yagita H, Omura K, Kasuga M. Prevention of autoimmune insulin-dependent diabetes in non-obese diabetic mice by anti-LFA1 and anti-ICAM1 mAb. Int Immunol. 1994;6:831–838. doi: 10.1093/intimm/6.6.831. [DOI] [PubMed] [Google Scholar]
- Hatada MH, Lu X, Laird ER, Green J, Morgenstern JP, Lou M, Marr CS, Phillips TB, Ram MK, Theriault K, Zoller MJ, Karas JL. Molecular basis for the interaction of the protein tyrosine kinase ZAP70 with the T cell receptor. Nature. 1995;377:32–38. doi: 10.1038/377032a0. [DOI] [PubMed] [Google Scholar]
- Isobe M, Yagita H, Okumura K, Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992;255:1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- Iwashima M, Irving BA, Van Oers NSC, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- Kanner SB, Grosmaire LS, Ledbetter JA, Damle NK. β2-integrin LFA-1 signaling through phospholipase C-γ1 activation. Proc Natl Acad Sci USA. 1993;90:7099–7103. doi: 10.1073/pnas.90.15.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely PJ, Parise LV. The α2β1 integrin is a necessary co-receptor for collagen-induced activation of Syk and the subsequent phosphorylation of phospholipase Cγ2 in platelets. J Biol Chem. 1996;271:26668–26676. [PubMed] [Google Scholar]
- Kong G, Dalton M, Bubeck J, Wardenburg, Straus D, Kurosaki T, Chan AC. Distinct tyrosine phosphorylation sites in ZAP-70 mediate activation and negative regulation of antigen receptor function. Mol Cell Biol. 1996;16:5026–5035. doi: 10.1128/mcb.16.9.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundra V, Escobedo JA, Kazlauskas A, Kim HK, Rhee SG, Williams LT, Zetter BR. Regulation of chemotaxis by the platelet-derived growth factor receptor-β. Nature. 1994;367:474–476. doi: 10.1038/367474a0. [DOI] [PubMed] [Google Scholar]
- La Rivière G, Schipper CA, Collard JG, Roos E. Invasiveness in hepatocyte and fibroblast monolayers and metastatic potential of T cell hybridomas in mice. Cancer Res. 1988;48:3405–3410. [PubMed] [Google Scholar]
- La Rivière G, Klein JWTM, Gebbink, Driessens MHE, Roos E. Pertussis toxin inhibition of T cell hybridoma invasion is reversed by manganese-induced activation of LFA-1. J Cell Sci. 1994;107:551–559. doi: 10.1242/jcs.107.3.551. [DOI] [PubMed] [Google Scholar]
- La Rivière G, Klein JWTM, Gebbinck, Schipper CA, Mooi WJ, Roos E. In vitro invasiveness of CTL clones and in vivo dissemination of CTL hybridomas. J Leukoc Biol. 1993;53:381–389. doi: 10.1002/jlb.53.4.381. [DOI] [PubMed] [Google Scholar]
- Lang V, Mege D, Semichon M, Gary-Gouy H, Bismuth G. A dual participation of ZAP-70 and Src protein tyrosine kinases is required for TCR induced tyrosine phosphorylation of Sam68 in Jurkat T cells. Eur J Immunol. 1997;27:3360–3367. doi: 10.1002/eji.1830271235. [DOI] [PubMed] [Google Scholar]
- Lin TH, Rosales C, Mondal K, Bolen JB, Haskill S, Juliano RL. Integrin-mediated tyrosine phosphorylation and cytokine message induction in monocytic cells. A possible signaling role for the Syk tyrosine kinase. J Biol Chem. 1995;270:16189–16197. doi: 10.1074/jbc.270.27.16189. [DOI] [PubMed] [Google Scholar]
- Lorant DE, Patel KD, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Coexpression of GMP140 and PAF by endothelium stimulated by histamine or thrombin: A juxtacrine system for adhesion and activation of neutrophils. JCell Biol. 1991;115:223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupher ML, Reedquist KA, Miyake S, Langdon WY, Band H. A novel phosphotyrosine-binding domain in the NH2-terminal transforming region of Cbl interacts directly and selectively with ZAP-70 in T cells. J Biol Chem. 1996;271:24063–24068. doi: 10.1074/jbc.271.39.24063. [DOI] [PubMed] [Google Scholar]
- Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- Michiels F, Habets GGM, Stam JC, van der Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Mountford P, Zevnik B, Duwel A, Nichols J, Li M, Dani C, Robertson M, Chambers I, Smith A. Dicistronic targeting constructs: reporters and modifiers of mammalian gene expression. Proc Natl Acad Sci USA. 1994;91:4303–4307. doi: 10.1073/pnas.91.10.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi I, Motoyama N, Nakayama K, Nakayama K, Senju S, Hatakeyama S, Zhang Q, Chan AC, Loh DY. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- Oliver JM, Burg DL, Wilson BS, McLaughlin JL, Geahlen RL. Inhibition of mast cell FceR1-mediated signaling and effector function by the Syk-selective inhibitor, piceatannol. J Biol Chem. 1994;269:29697–29703. [PubMed] [Google Scholar]
- Olson MF, Pasteris NG, Gorski JL, Hall A. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr Biol. 1996;6:1628–1633. doi: 10.1016/s0960-9822(02)70786-0. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D, Mollenauer MN, Weiss A. Dominant-negative Zeta-associated protein 70 inhibits T cell antigen receptor signaling. J Exp Med. 1996;183:611–620. doi: 10.1084/jem.183.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos E, La Rivière G, Collard JG, Stukart MJ, De Baetselier P. Invasiveness of T cell hybridomas in vitro and their metastatic potential in vivo. Cancer Res. 1985;45:6238–6243. [PubMed] [Google Scholar]
- Roos E, Van de Pavert IV. Inhibition of lymphoma invasion and liver metastasis formation by pertussis toxin. Cancer Res. 1987;47:5439–5444. [PubMed] [Google Scholar]
- Roossien FF, De Rijk D, Bikker A, Roos E. Involvement of LFA-1 in lymphoma invasion and metastasis demonstrated with LFA-1–deficient mutants. J Cell Biol. 1989;108:1979–1985. doi: 10.1083/jcb.108.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F, Simon P, Thompson S, Springer TA. Mapping of antigenic and functional epitopes on the α- and β-subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983;158:586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-ζ and lack of ZAP-70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J, Zhang W, Presley J, Williams BL, Abraham RT, Lippincott-Schwartz J, Samelson LE. Regulation of ZAP-70 intracellular localization: Visualization with the green fluorescent protein. J Exp Med. 1997;186:1713–1724. doi: 10.1084/jem.186.10.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit L, van der Horst G, Borst J. Sos, Vav, and C3G participate in B cell receptor-induced signaling pathways and differentially associate with Shc-Grb2, Crk, and Crk-L adaptors. J Biol Chem. 1996;271:8564–8569. doi: 10.1074/jbc.271.15.8564. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Hogervorst F, Osterop A, Veltman FEM. Identification and characterization of a novel antigen complex on mouse mammary tumor cells using a monoclonal antibody against platelet glycoprotein Ic. J Biol Chem. 1988;263:14030–14038. [PubMed] [Google Scholar]
- Staal FJT, Bakker AQ, Verkuijlen M, van Oort E, Spits H. Use of bicistronic retroviral vectors encoding the LacZ gene with a gene of interest: A method to select producer cells and follow transduced target cells. Cancer Gene Ther. 1996;3:345–351. [PubMed] [Google Scholar]
- Sugie K, Minami Y, Kawakami T, Uchida A. Stimulation of NKlike YT cells via leukocyte function-associated antigen (LFA)1. Possible involvement of LFA-1 associated tyrosine kinase in signal transduction after recognition of NK target cells. J Immunol. 1995;154:1691–1698. [PubMed] [Google Scholar]
- Sunder-Plassmann R, Lialios F, Madsen M, Koyasu S, Reinherz EL. Functional analysis of immunoreceptor tyrosine-based activation motif (ITAM)-mediated signal transduction: the two YxxL segments within a single CD3ζ-ITAM are functionally distinct. Eur J Immunol. 1997;27:2001–2009. doi: 10.1002/eji.1830270826. [DOI] [PubMed] [Google Scholar]
- Tashiro K, Hideaki T, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: A cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- Wan Y, Kurosaki T, Huang XY. Tyrosine kinases in activation of the MAP kinase cascade by G protein-coupled receptors. Nature. 1996;380:541–544. doi: 10.1038/380541a0. [DOI] [PubMed] [Google Scholar]
- Weil R, Cloutier JF, Fournel M, Veillette A. Regulation of ZAP-70 by Src family tyrosine protein kinases in an antigen-specific T cell line. J Biol Chem. 1995;270:2791–2799. doi: 10.1074/jbc.270.6.2791. [DOI] [PubMed] [Google Scholar]
- Welder CA, Lee DHS, Takei F. Inhibition of cell adhesion by microspheres coated with recombinant soluble intercellular adhesion molecule-1. J Immunol. 1993;150:2203–2210. [PubMed] [Google Scholar]
- Wennström S, Sieghbahn A, Yokote K, Arvidsson AK, Heldin CH, Mori S, Claesson-Welsh L. Membrane ruffling and chemotaxis transduced by the PDGF β-receptor require the binding site for phosphatidylinositol 3' kinase. Oncogene. 1994;9:651–660. [PubMed] [Google Scholar]
- Wu J, Zhao T, Kurosaki T, Weiss A. The Vav binding site (Y315) in ZAP-70 is critical for antigen receptor-mediated signal transduction. J Exp Med. 1997;185:1877–1882. doi: 10.1084/jem.185.10.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SR, Huang M, Berton G. Signaling by adhesion in human neutrophils. Activation of the p72syk tyrosine kinase and formation of protein complexes containing p72sykand Src family kinases in neutrophils spreading over fibrinogen. J Immunol. 1997;158:1902–1910. [PubMed] [Google Scholar]
- Zheng L, Sjolander A, Eckerdal J, Andersson T. Antibody-induced engagement of β2 integrins on adherent human neutrophils triggers activation of p21rasthrough tyrosine phosphorylation of the protooncogene product Vav. Proc Natl Acad Sci USA. 1996;93:8431–8436. doi: 10.1073/pnas.93.16.8431. [DOI] [PMC free article] [PubMed] [Google Scholar]