Abstract
We are synthesizing and testing various 2-nitroimidazole analogs with different octanol/water partition coefficients for their radiosensitizing and cytototoxic properties. Our hypothesis is that, unlike the situation in vitro, lipophilicity will play a major role in determining the extent of radiosensitization of hypoxic tumour cells in vivo and also the degree of animal toxicity. The radiosensitivity testing involves determination of the surviving fraction of EMT6 tumour cells irradiated in vivo under different conditions following drug injection, and subsequent assay in vitro. Data obtained to date on eight 2-nitroimidazoles do not indicate any compound clearly superior to misonidazole (Ro-07-0582), although one or two are close and may prove better at the completion of testing. Cytotoxicity of the compounds is assayed by cell survival and histologically. Data obtained on the toxicity of misonidazole to the cells of EMT6 tumours suggest that a large proportion of the anoxic cells in these tumours are not near necrotic areas, but rather result from the temporary closure of blood vessels.
Full text
PDF
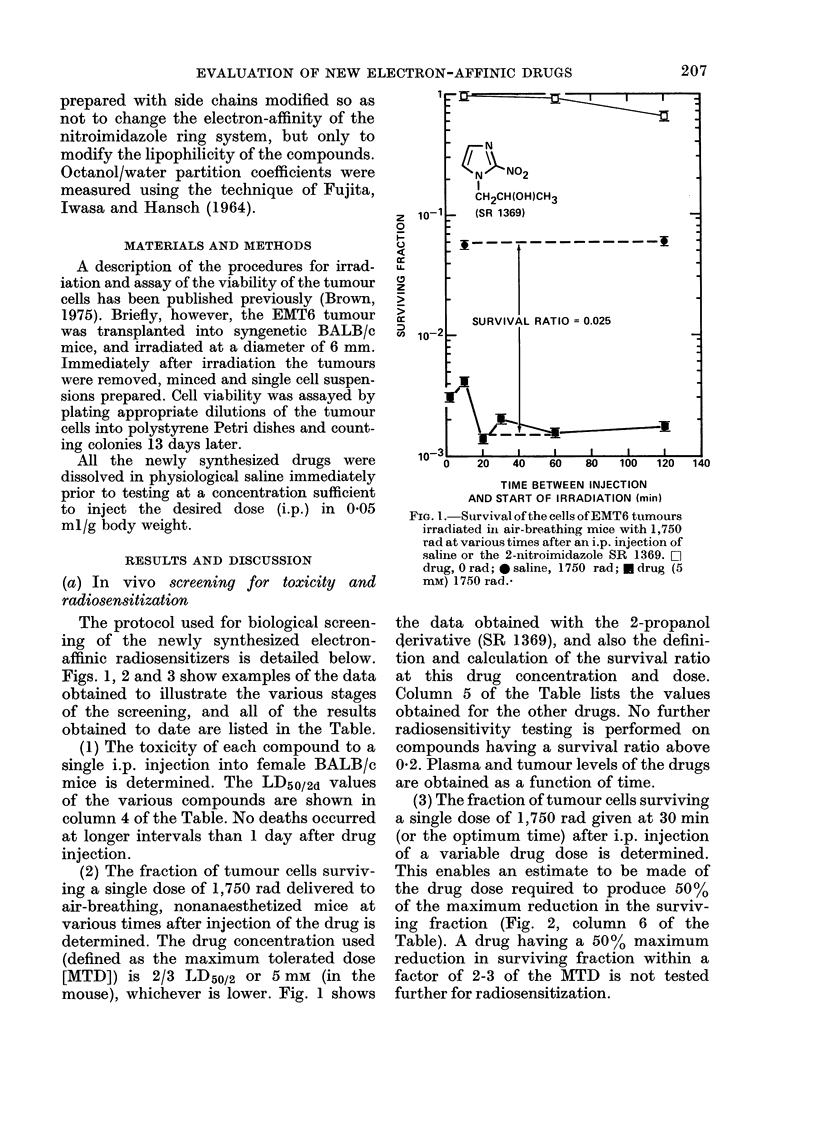
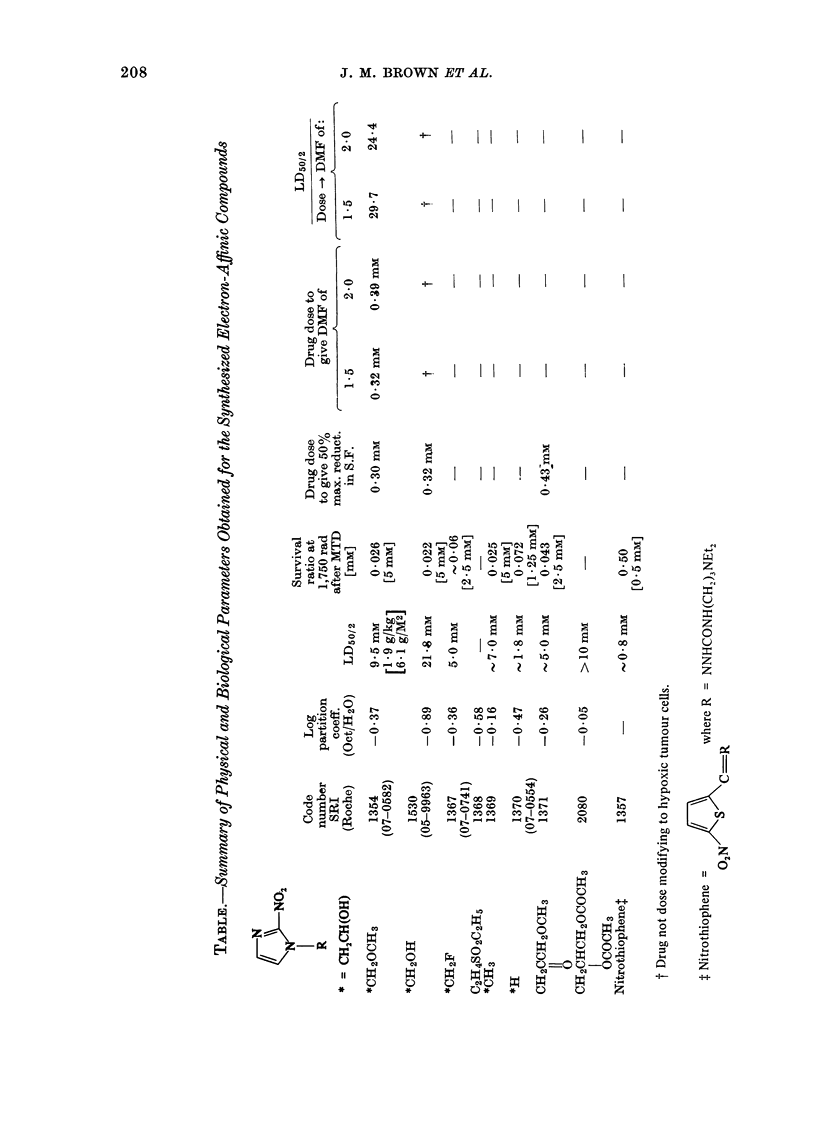
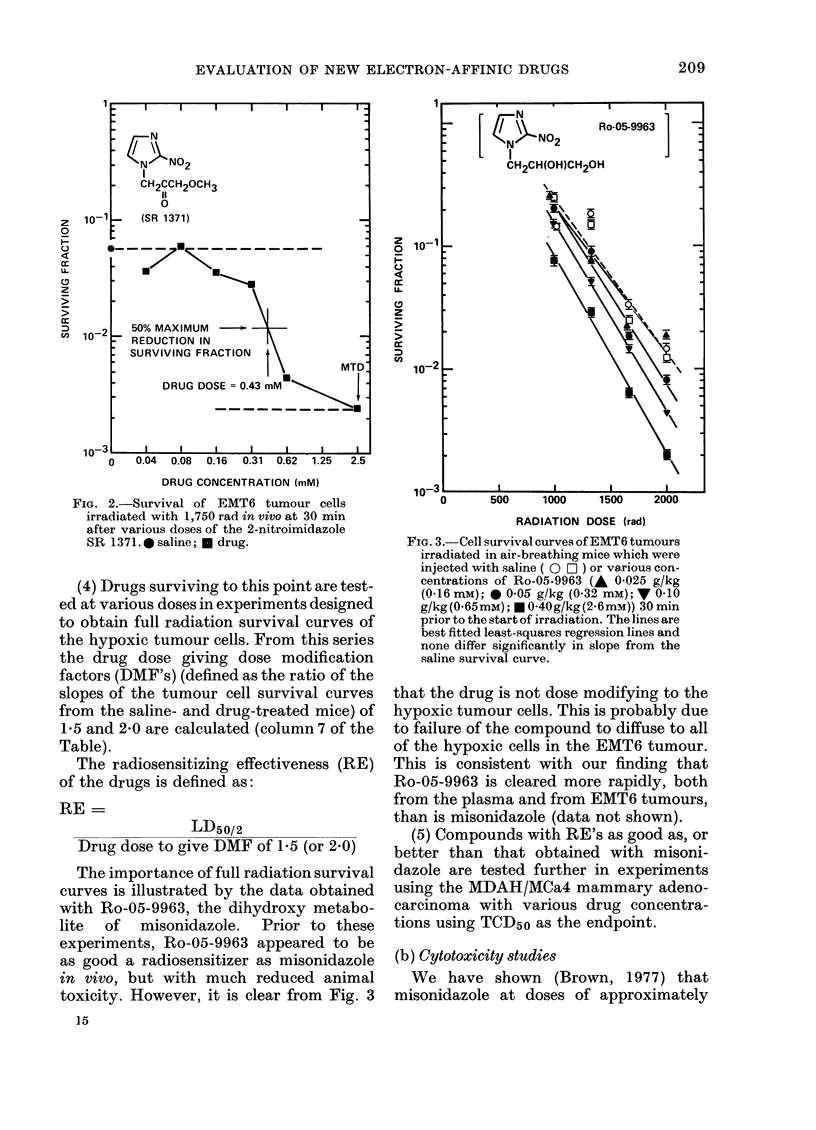
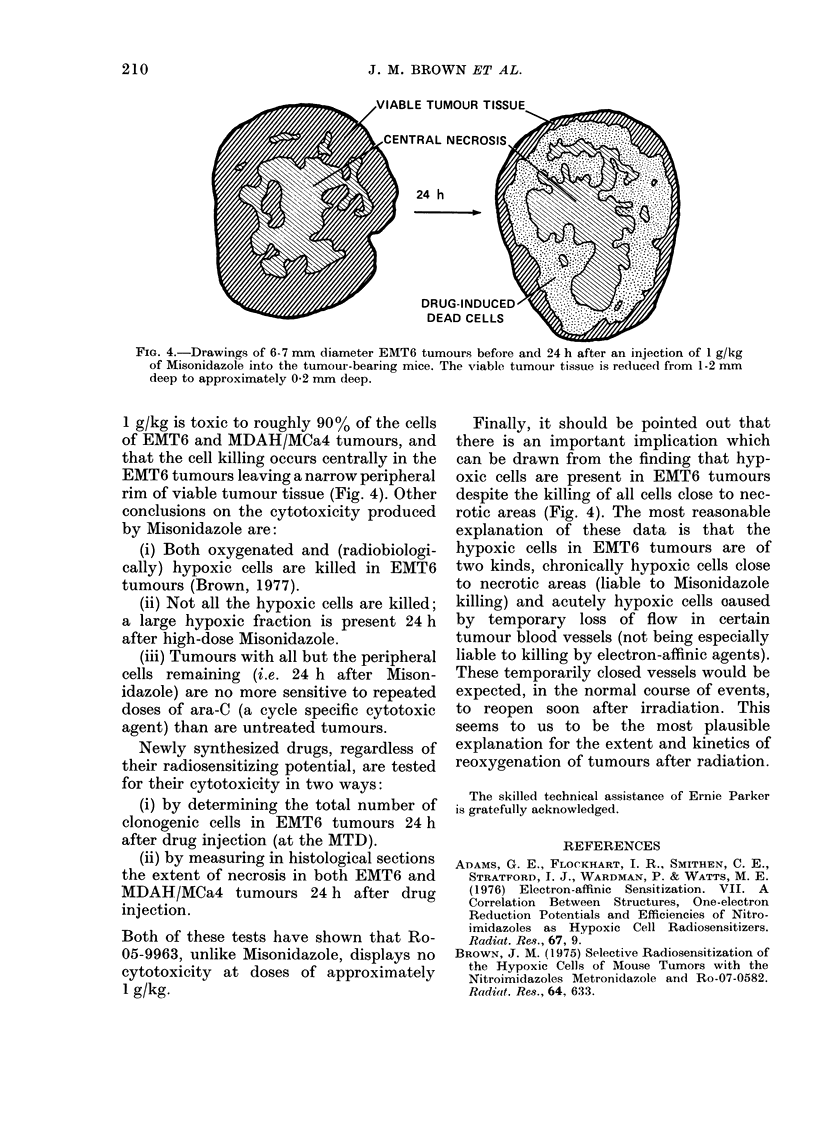

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E., Flockhart I. R., Smithen C. E., Stratford I. J., Wardman P., Watts M. E. Electron-affinic sensitization. VII. A correlation between structures, one-electron reduction potentials, and efficiencies of nitroimidazoles as hypoxic cell radiosensitizers. Radiat Res. 1976 Jul;67(1):9–20. [PubMed] [Google Scholar]
- Brown J. M. Cytotoxic effects of the hypoxic cell radiosensitizer Ro 7-0582 to tumor cells in vivo. Radiat Res. 1977 Dec;72(3):469–486. [PubMed] [Google Scholar]
- Brown J. M. Selective radiosensitization of the hypoxic cells of mouse tumors with the nitroimidazoles metronidazole and Ro 7-0582. Radiat Res. 1975 Dec;64(3):633–647. [PubMed] [Google Scholar]
- Hansch C., Clayton J. M. Lipophilic character and biological activity of drugs. II. The parabolic case. J Pharm Sci. 1973 Jan;62(1):1–21. doi: 10.1002/jps.2600620102. [DOI] [PubMed] [Google Scholar]
- Kubinyi H. Quantitative structure--activity relationships. 7. The bilinear model, a new model for nonlinear dependence of biological activity on hydrophobic character. J Med Chem. 1977 May;20(5):625–629. doi: 10.1021/jm00215a002. [DOI] [PubMed] [Google Scholar]


