Abstract
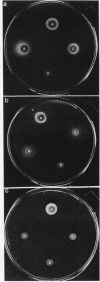
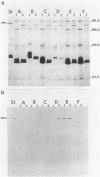
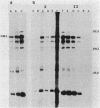
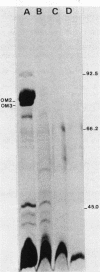
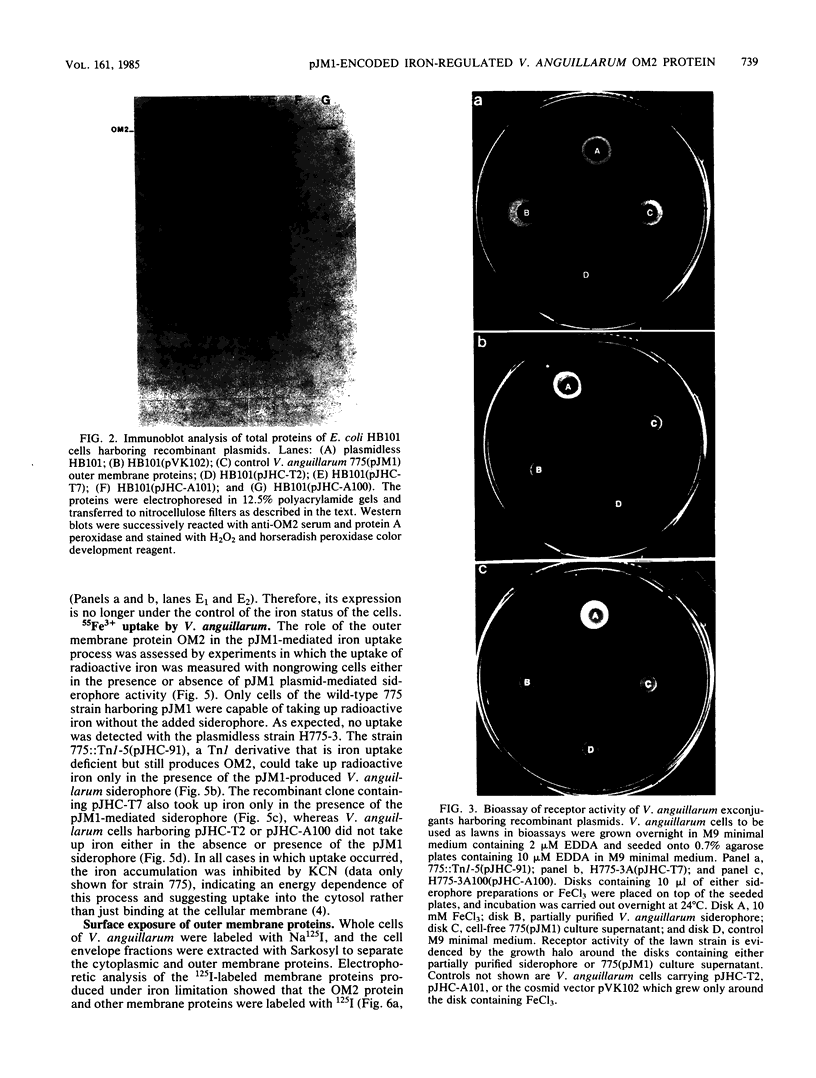
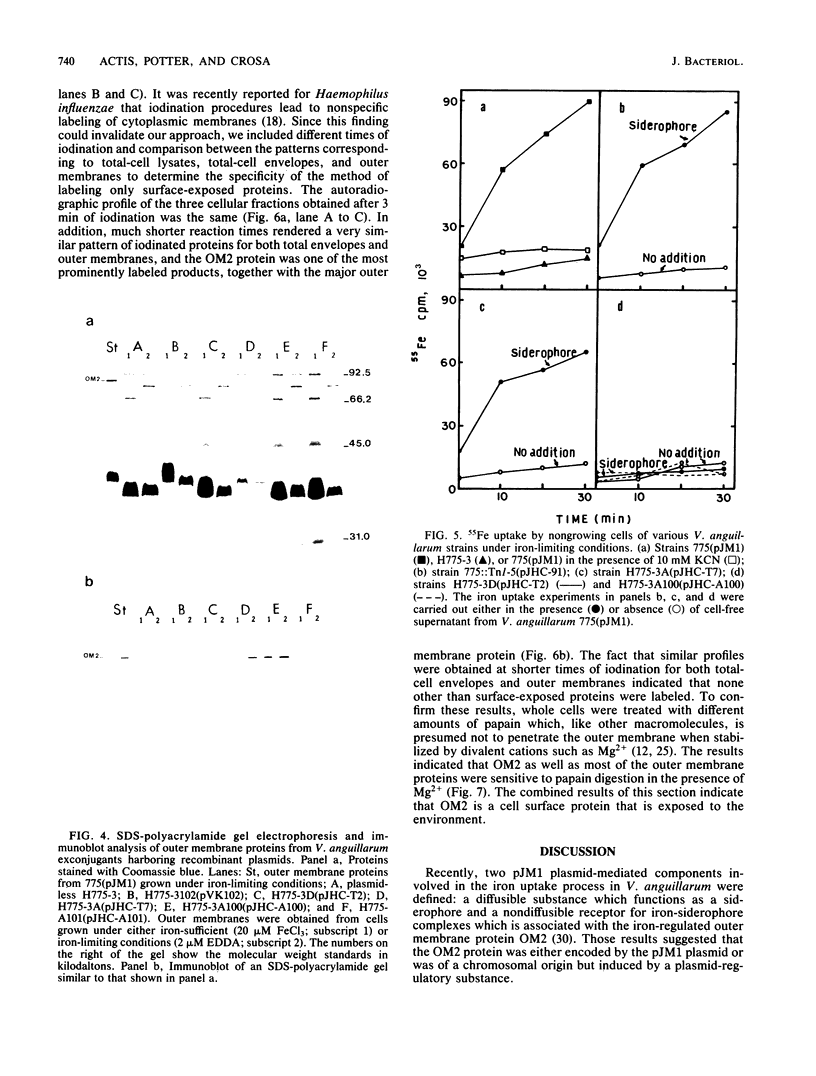
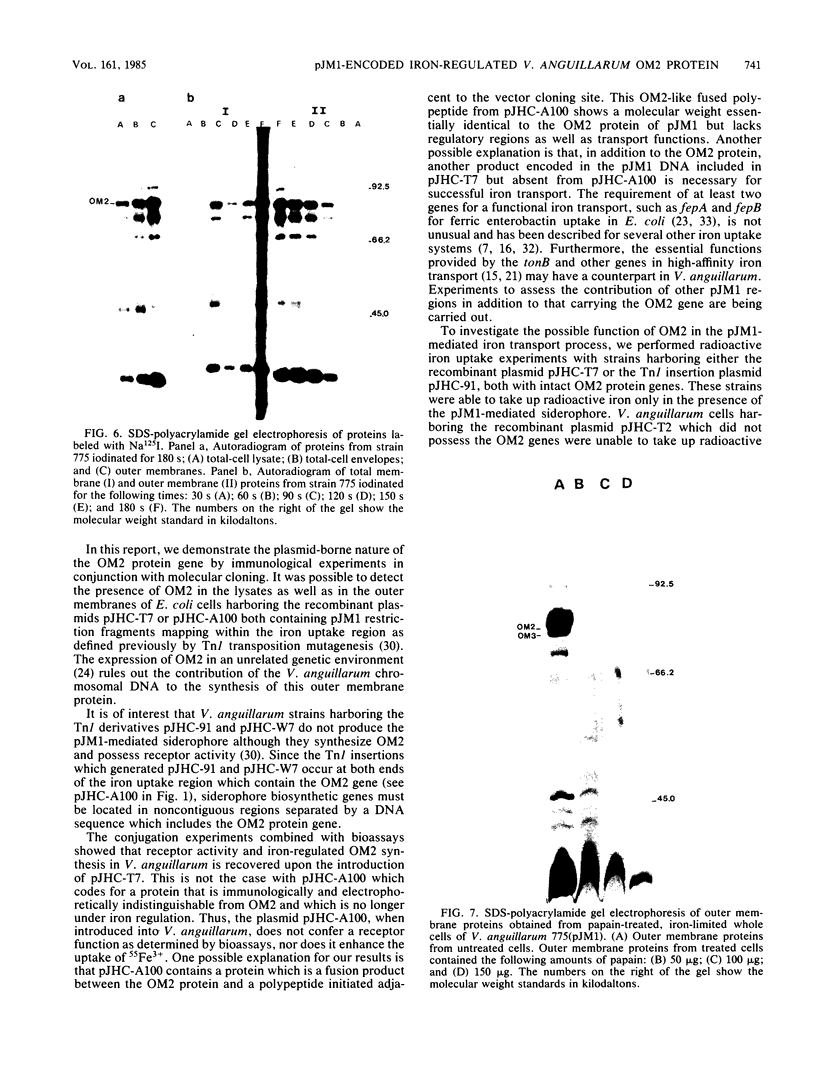
Vibrio anguillarum 775 harboring the virulence plasmid pJM1 synthesized an outer membrane protein of 86 kilodaltons, OM2, that was inducible under conditions of iron limitation. pJM1 DNA fragments obtained by digestion with restriction endonucleases were cloned into cosmid vectors and transferred into Escherichia coli. The OM2 protein was synthesized in E. coli, demonstrating that it is actually encoded by the pJM1 plasmid. Mobilization of the recombinant plasmids to V. anguillarum was accomplished by using the transfer factor pRK2013. A V. anguillarum exconjugant harboring the recombinant derivative pJHC-T7 and synthesizing the OM2 protein took up 55Fe3+ and grew under iron-limiting conditions, only in presence of the pJM1-mediated siderophore. Exconjugants harboring recombinant plasmids, such as pJHC-T2 which did not encode the OM2 protein, were transport negative. Membrane protein iodination experiments, together with protease treatment of whole cells, indicated that the OM2 protein is exposed to the outside environment of the V. anguillarum cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Cox C. D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980 May;142(2):581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature. 1980 Apr 10;284(5756):566–568. doi: 10.1038/284566a0. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Hodges L. L. Outer membrane proteins induced under conditions of iron limitation in the marine fish pathogen Vibrio anguillarum 775. Infect Immun. 1981 Jan;31(1):223–227. doi: 10.1128/iai.31.1.223-227.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Hodges L. L., Schiewe M. H. Curing of a plasmid is correlated with an attenuation of virulence in the marine fish pathogen Vibrio anguillarum. Infect Immun. 1980 Mar;27(3):897–902. doi: 10.1128/iai.27.3.897-902.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Falkow S. Molecular cloning of replication and incompatibility regions from the R-plasmid R6K. J Mol Biol. 1978 Sep 25;124(3):443–468. doi: 10.1016/0022-2836(78)90181-x. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Schiewe M. H., Falkow S. Evidence for plasmid contribution to the virulence of fish pathogen Vibrio anguillarum. Infect Immun. 1977 Nov;18(2):509–513. doi: 10.1128/iai.18.2.509-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. The relationship of plasmid-mediated iron transport and bacterial virulence. Annu Rev Microbiol. 1984;38:69–89. doi: 10.1146/annurev.mi.38.100184.000441. [DOI] [PubMed] [Google Scholar]
- Di Rienzo J. M., Spieler E. L. Identification and characterization of the major cell envelope proteins of oral strains of Actinobacillus actinomycetemcomitans. Infect Immun. 1983 Jan;39(1):253–261. doi: 10.1128/iai.39.1.253-261.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein S., Hantke K., Braun V. Citrate-dependent iron transport system in Escherichia coli K-12. Eur J Biochem. 1981 Jul;117(2):431–437. doi: 10.1111/j.1432-1033.1981.tb06357.x. [DOI] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Lactoperoxidase and Iodo-Gen-catalyzed iodination labels inner and outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Jul;155(1):443–446. doi: 10.1128/jb.155.1.443-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. The critical role of iron in host-bacterial interactions. J Clin Invest. 1978 Jun;61(6):1428–1440. doi: 10.1172/JCI109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. R., Pickett C. L., Earhart C. F. Two fep genes are required for ferrienterochelin uptake in Escherichia coli K-12. J Bacteriol. 1983 Jul;155(1):330–336. doi: 10.1128/jb.155.1.330-336.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiewe M. H., Crosa J. H., Ordal E. J. Deoxyribonucleic acid relationships among marine vibrios pathogenic to fish. Can J Microbiol. 1977 Aug;23(8):954–958. doi: 10.1139/m77-142. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. H., Williams R. P. Use of iodo-gen and iodine-125 to label the outer membrane proteins of whole cells of Neisseria gonorrhoeae. Anal Biochem. 1982 Mar 1;120(2):254–258. doi: 10.1016/0003-2697(82)90344-x. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Rodriguez R. L., West R. W., Jr The rapid purification of T4 DNA ligase from a lambda T4 lig lysogen. J Biol Chem. 1980 Feb 10;255(3):813–815. [PubMed] [Google Scholar]
- Tolmasky M. E., Crosa J. H. Molecular cloning and expression of genetic determinants for the iron uptake system mediated by the Vibrio anguillarum plasmid pJM1. J Bacteriol. 1984 Dec;160(3):860–866. doi: 10.1128/jb.160.3.860-866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. A., Potter S. A., Crosa J. H. Iron uptake system medicated by Vibrio anguillarum plasmid pJM1. J Bacteriol. 1983 Nov;156(2):880–887. doi: 10.1128/jb.156.2.880-887.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wookey P. J., Hussein S., Braun V. Functions in outer and inner membranes of Escherichia coli for ferrichrome transport. J Bacteriol. 1981 Jun;146(3):1158–1161. doi: 10.1128/jb.146.3.1158-1161.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wookey P., Rosenberg H. Involvement of inner and outer membrane components in the transport of iron and in colicin B action in Escherichia coli. J Bacteriol. 1978 Feb;133(2):661–666. doi: 10.1128/jb.133.2.661-666.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]