Abstract
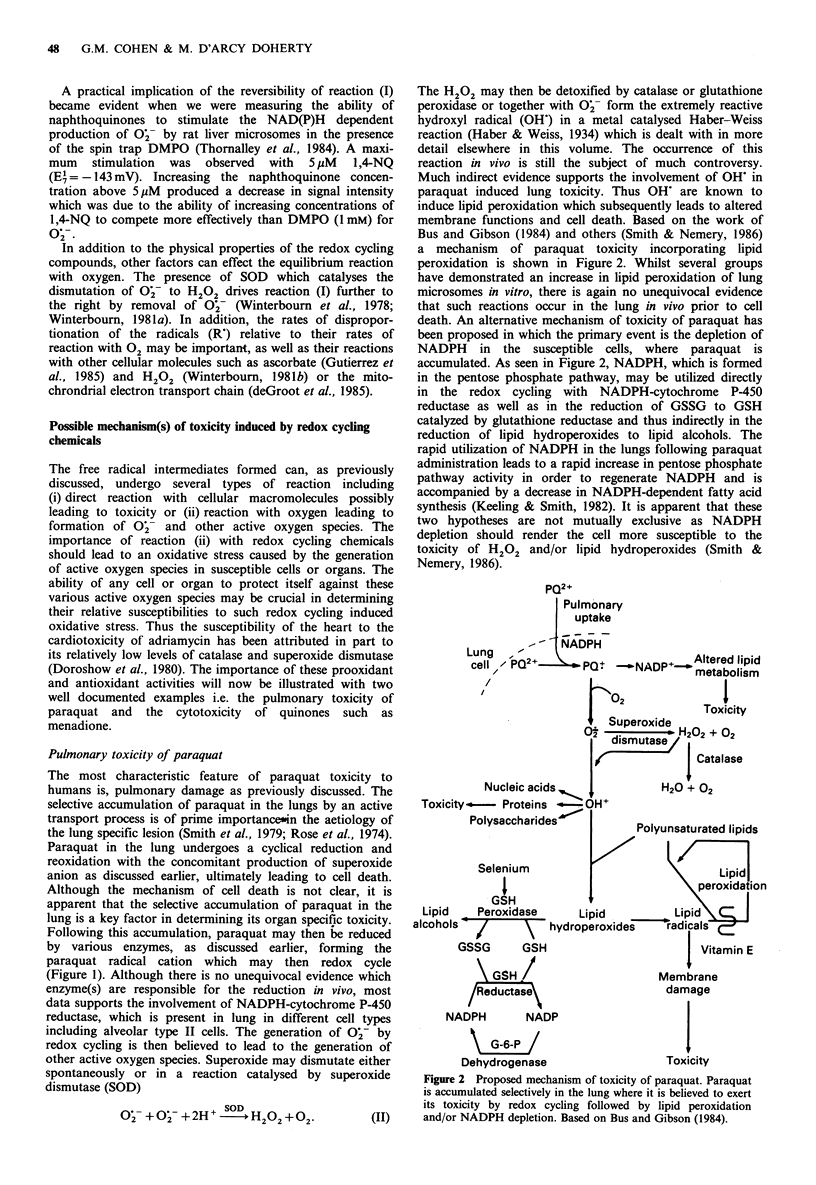
Free radical formation has been implicated in the toxicity of a wide range of xenobiotics. In recent years, particular interest has been paid to compounds which can undergo a one electron reduction to form a radical species which can then react with oxygen forming superoxide (O2.-) and regenerating the parent molecule. This process, which is called redox cycling, leads to a disproportionate consumption of O2 and cellular reducing equivalents and the formation of active oxygen species, ultimately causing oxidative stress. It has been proposed that cell death results from a loss in control of Ca2+ homeostasis caused by thiol oxidation at critical enzyme sites. Physical properties of redox cycling compounds such as their one electron reduction potentials are important in determining their rate of reduction by cellular reductases and the reactivity of the radicals so formed with oxygen and other molecules. Although redox cycling of many compounds can be clearly demonstrated in vitro, the unequivocal demonstration of this process in vivo and its involvement in in vivo toxicities remains a challenging area for future research.
Full text
PDF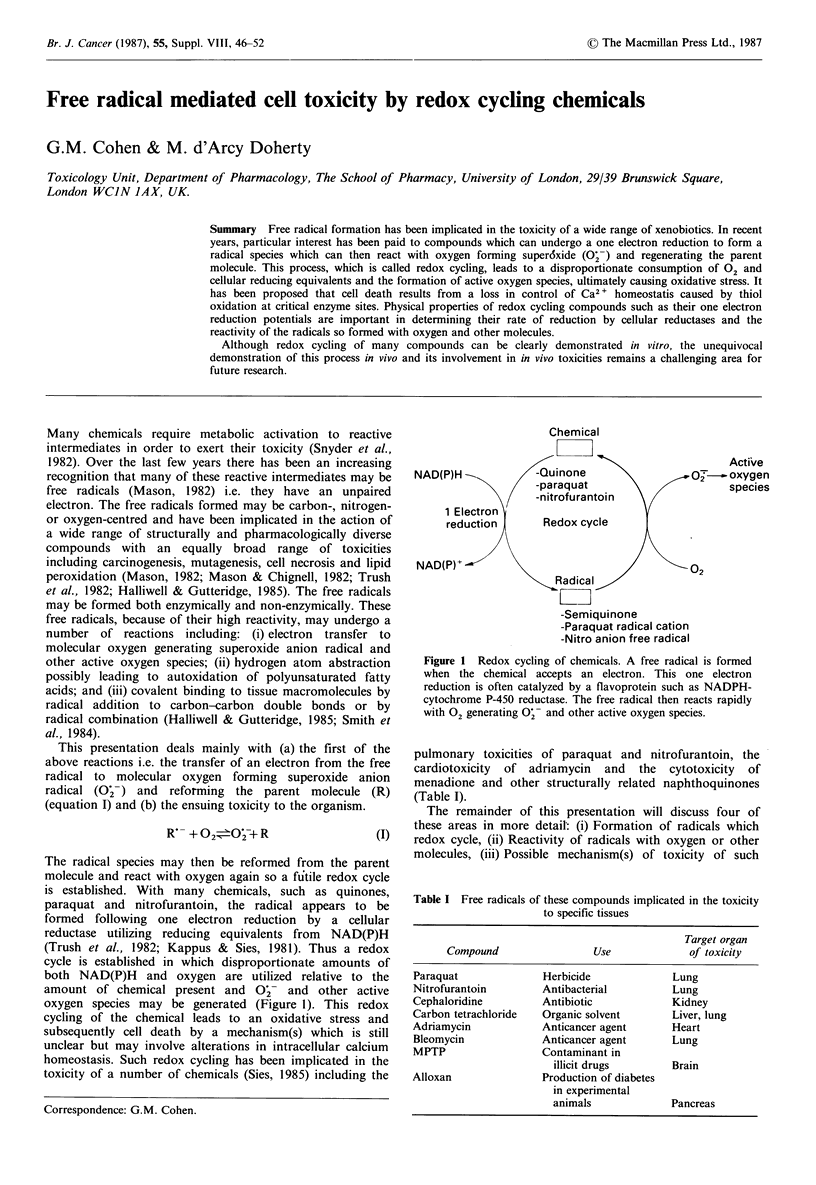



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bus J. S., Gibson J. E. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984 Apr;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chignell C. F., Motten A. G., Buettner G. R. Photoinduced free radicals from chlorpromazine and related phenothiazines: relationship to phenothiazine-induced photosensitization. Environ Health Perspect. 1985 Dec;64:103–110. doi: 10.1289/ehp.8564103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Monte D., Bellomo G., Thor H., Nicotera P., Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch Biochem Biophys. 1984 Dec;235(2):343–350. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- Di Monte D., Ross D., Bellomo G., Eklöw L., Orrenius S. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch Biochem Biophys. 1984 Dec;235(2):334–342. doi: 10.1016/0003-9861(84)90206-6. [DOI] [PubMed] [Google Scholar]
- Doherty M. A., Makowski R., Gibson G. G., Cohen G. M. Cytochrome P-450 dependent metabolic activation of 1-naphthol to naphthoquinones and covalent binding species. Biochem Pharmacol. 1985 Jul 1;34(13):2261–2267. doi: 10.1016/0006-2952(85)90779-8. [DOI] [PubMed] [Google Scholar]
- Doroshow J. H., Locker G. Y., Myers C. E. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980 Jan;65(1):128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez P. L., Egorin M. J., Davis T. A., Bachur N. R. Effects of ascorbic acid on biologically obtained diaziquone free radicals. Biochem Pharmacol. 1985 Jul 1;34(13):2394–2397. doi: 10.1016/0006-2952(85)90802-0. [DOI] [PubMed] [Google Scholar]
- Handa K., Sato S. Generation of free radicals of quinone group-containing anti-cancer chemicals in NADPH-microsome system as evidenced by initiation of sulfite oxidation. Gan. 1975 Feb;66(1):43–47. [PubMed] [Google Scholar]
- Jewell S. A., Bellomo G., Thor H., Orrenius S., Smith M. Bleb formation in hepatocytes during drug metabolism is caused by disturbances in thiol and calcium ion homeostasis. Science. 1982 Sep 24;217(4566):1257–1259. doi: 10.1126/science.7112127. [DOI] [PubMed] [Google Scholar]
- Kappus H. Overview of enzyme systems involved in bio-reduction of drugs and in redox cycling. Biochem Pharmacol. 1986 Jan 1;35(1):1–6. doi: 10.1016/0006-2952(86)90544-7. [DOI] [PubMed] [Google Scholar]
- Kappus H., Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981 Dec 15;37(12):1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Kubow S., Janzen E. G., Bray T. M. Spin-trapping of free radicals formed during in vitro and in vivo metabolism of 3-methylindole. J Biol Chem. 1984 Apr 10;259(7):4447–4451. [PubMed] [Google Scholar]
- Lind C., Hochstein P., Ernster L. DT-diaphorase as a quinone reductase: a cellular control device against semiquinone and superoxide radical formation. Arch Biochem Biophys. 1982 Jun;216(1):178–185. doi: 10.1016/0003-9861(82)90202-8. [DOI] [PubMed] [Google Scholar]
- Mason R. P., Chignell C. F. Free radicals in pharmacology and toxicology--selected topics. Pharmacol Rev. 1981 Dec;33(4):189–211. [PubMed] [Google Scholar]
- Miller M. G., Rodgers A., Cohen G. M. Mechanisms of toxicity of naphthoquinones to isolated hepatocytes. Biochem Pharmacol. 1986 Apr 1;35(7):1177–1184. doi: 10.1016/0006-2952(86)90157-7. [DOI] [PubMed] [Google Scholar]
- Muir Wood P. The redox potential of the system oxygen--superoxide. FEBS Lett. 1974 Aug 15;44(1):22–24. doi: 10.1016/0014-5793(74)80297-8. [DOI] [PubMed] [Google Scholar]
- Poyer J. L., McCay P. B., Weddle C. C., Downs P. E. In vivo spin-trapping of radicals formed during halothane metabolism. Biochem Pharmacol. 1981 Jun 15;30(12):1517–1519. doi: 10.1016/0006-2952(81)90375-0. [DOI] [PubMed] [Google Scholar]
- Rose M. S., Smith L. L., Wyatt I. Evidence for energy-dependent accumulation of paraquat into rat lung. Nature. 1974 Nov 22;252(5481):314–315. doi: 10.1038/252314b0. [DOI] [PubMed] [Google Scholar]
- Smith C. V., Hughes H., Mitchell J. R. Free radicals in vivo. Covalent binding to lipids. Mol Pharmacol. 1984 Jul;26(1):112–116. [PubMed] [Google Scholar]
- Smith P., Heath D. Paraquat. CRC Crit Rev Toxicol. 1976 Oct;4(4):411–445. doi: 10.1080/10408447609164020. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Thornalley P. J., Doherty M. D., Smith M. T., Bannister J. V., Cohen G. M. The formation of active oxygen species following activation of 1-naphthol, 1,2- and 1,4-naphthoquinone by rat liver microsomes. Chem Biol Interact. 1984 Feb;48(2):195–206. doi: 10.1016/0009-2797(84)90121-2. [DOI] [PubMed] [Google Scholar]
- Trush M. A., Mimnaugh E. G., Gram T. E. Activation of pharmacologic agents to radical intermediates. Implications for the role of free radicals in drug action and toxicity. Biochem Pharmacol. 1982 Nov 1;31(21):3335–3346. doi: 10.1016/0006-2952(82)90609-8. [DOI] [PubMed] [Google Scholar]
- Wardman P., Clarke E. D. Oxygen inhibition of nitroreductase: electron transfer from nitro radical-anions to oxygen. Biochem Biophys Res Commun. 1976 Apr 19;69(4):942–949. doi: 10.1016/0006-291x(76)90464-2. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Cytochrome c reduction by semiquinone radicals can be indirectly inhibited by superoxide dismutase. Arch Biochem Biophys. 1981 Jun;209(1):159–167. doi: 10.1016/0003-9861(81)90268-x. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., French J. K., Claridge R. F. Superoxide dismutase as an inhibitor of reactions of semiquinone radicals. FEBS Lett. 1978 Oct 15;94(2):269–272. doi: 10.1016/0014-5793(78)80953-3. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Production of hydroxyl radicals from paraquat radicals and H2O2. FEBS Lett. 1981 Jun 15;128(2):339–342. doi: 10.1016/0014-5793(81)80112-3. [DOI] [PubMed] [Google Scholar]
- Yamazaki I., Tamura M., Nakajima R., Nakamura M. Physiological aspects of free-radical reactions. Environ Health Perspect. 1985 Dec;64:331–342. doi: 10.1289/ehp.8564331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot H., Noll T., Sies H. Oxygen dependence and subcellular partitioning of hepatic menadione-mediated oxygen uptake. Studies with isolated hepatocytes, mitochondria, and microsomes from rat liver in an oxystat system. Arch Biochem Biophys. 1985 Dec;243(2):556–562. doi: 10.1016/0003-9861(85)90532-6. [DOI] [PubMed] [Google Scholar]


