Abstract
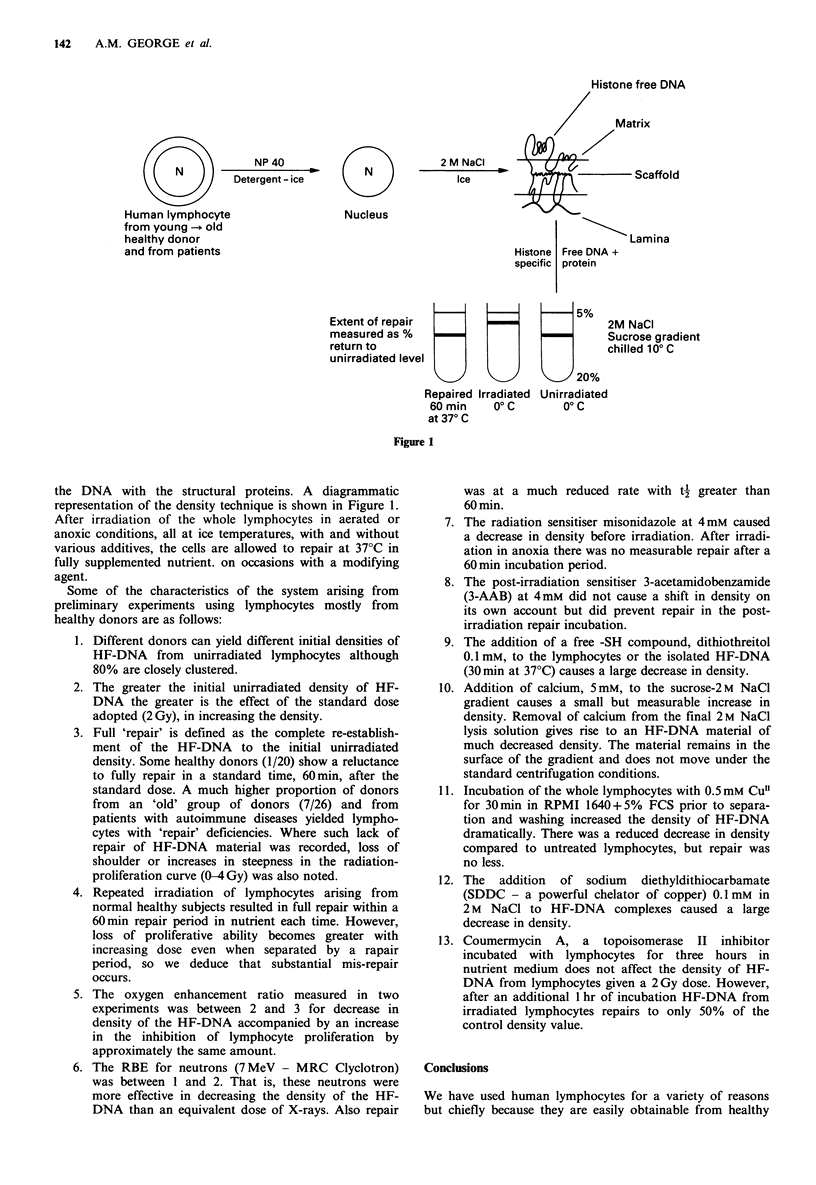
On challenging with 2M NaCl, the nuclei of human lymphocytes yield an aggregate of DNA-protein material. The density of the material is less when isolated from irradiated cells than when isolated from unirradiated cells. The density of this material, designated histone-free-DNA (HF-DNA), from irradiated cells returns to that from unirradiated cells if the irradiated cells are allowed time at 37 degrees C in nutrient conditions. Lymphocyte HF-DNA from patients who have exhibited hypersensitivity to radiotherapy exhibit slower repair characteristics than lymphocyte HF-DNA from the average normal subjects. Neutrons are more effective than X-rays in producing density changes. Misonidazole and the ADPRT inhibitor 3-AAB substantially inhibit return to unirradiated densities. The oer for the initial effect is between 2 and 3. These properties of this DNA material from human lymphocytes suggest that alterations in the configuration of such material by the loss and re-establishment of relatively weak cellular bonds are closely related to the well-known changes observed in classical cell survival experiments. Where the proliferation of human lymphocytes has been observed by concanavalin A stimulation, oer, RBE and chemical modification has been of the same order as the measured density changes. Additionally, we have found that the density of HF-DNA is heavily dependent upon Cu content. This has led us to propose that cell killing or malfunction at the nuclear level caused by ionizing radiation is caused by the conversion CuII----CuI and also by specific .OH attack on DNA or proteins at a Cu site.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPER T. LETHAL MUTATIONS AND CELL DEATH. Phys Med Biol. 1963 Nov;8:365–385. doi: 10.1088/0031-9155/8/4/301. [DOI] [PubMed] [Google Scholar]
- George A. M., Lunec J., Cramp W. A. Effect of membrane fatty acid changes on the radiation sensitivity of human lymphoid cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Apr;43(4):363–378. doi: 10.1080/09553008314550431. [DOI] [PubMed] [Google Scholar]
- Harris G., Cramp W. A., Edwards J. C., George A. M., Sabovljev S. A., Hart L., Hughes G. R., Denman A. M., Yatvin M. B. Radiosensitivity of peripheral blood lymphocytes in autoimmune disease. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Jun;47(6):689–699. doi: 10.1080/09553008514550931. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Laemmli U. K. Evidence for two levels of DNA folding in histone-depleted HeLa interphase nuclei. J Mol Biol. 1982 Apr 5;156(2):309–324. doi: 10.1016/0022-2836(82)90331-x. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Laemmli U. K. Non-histone proteins and long-range organization of HeLa interphase DNA. J Mol Biol. 1982 Apr 5;156(2):325–344. doi: 10.1016/0022-2836(82)90332-1. [DOI] [PubMed] [Google Scholar]
- Lewis C. D., Laemmli U. K. Higher order metaphase chromosome structure: evidence for metalloprotein interactions. Cell. 1982 May;29(1):171–181. doi: 10.1016/0092-8674(82)90101-5. [DOI] [PubMed] [Google Scholar]
- Lewis C. D., Lebkowski J. S., Daly A. K., Laemmli U. K. Interphase nuclear matrix and metaphase scaffolding structures. J Cell Sci Suppl. 1984;1:103–122. doi: 10.1242/jcs.1984.supplement_1.8. [DOI] [PubMed] [Google Scholar]
- Michael B. D., Adams G. E., Hewitt H. B., Jones W. B., Watts M. E. A posteffect of oxygen in irradiated bacteria: a submillisecond fast mixing study. Radiat Res. 1973 May;54(2):239–251. [PubMed] [Google Scholar]
- Samuni A., Chevion M., Czapski G. Unusual copper-induced sensitization of the biological damage due to superoxide radicals. J Biol Chem. 1981 Dec 25;256(24):12632–12635. [PubMed] [Google Scholar]
- Van Hemmen J. J., Meuling W. J. Inactivation of biologically active DNA by gamma-ray-induced superoxide radicals and their dismutation products singlet molecular oxygen and hydrogen peroxide. Biochim Biophys Acta. 1975 Aug 21;402(2):133–141. doi: 10.1016/0005-2787(75)90031-3. [DOI] [PubMed] [Google Scholar]
- Westman G., Midander J. Post-irradiation diethyldithiocarbamate-inhibition of CuZn superoxide dismutase reduces clonogenic survival of Chinese hamster V-79 cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Jan;45(1):11–20. doi: 10.1080/09553008414550021. [DOI] [PubMed] [Google Scholar]
- Wolters H., Konings A. W. Radiation effects on membranes. III. The effect of X irradiation on survival of mammalian cells substituted by polyunsaturated fatty acids. Radiat Res. 1982 Dec;92(3):474–482. [PubMed] [Google Scholar]
- Yatvin M. B., Gipp J. J., Dennis W. H. Influence of unsaturated fatty acids, membrane fluidity and oxygenation on the survival of an E. coli fatty acid auxotroph following gamma-irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1979 Jun;35(6):539–548. doi: 10.1080/09553007914550651. [DOI] [PubMed] [Google Scholar]



