Abstract
Maturation of physiologic process which govern the disposition of pharmacologic agents can yield significant changes in absorption, distribution, metabolism, and elimination of drugs in neonates, infants and children. However, there are very little data concerning the disposition of anticancer drugs in young children. Pharmacokinetic data for six anticancer agents were compared in infants less than 1 year of age and children greater than 1 year of age treated at St Jude Children's Research Hospital. No pharmacokinetic data were available for infants less than 2 months of age. Median methotrexate clearance tended to be lower in four infants (0.26-0.99 years) vs 108 children (1-19 years): 80 vs 103 ml min-1 m-2, respectively (P = 0.01). There was no difference in the median 42 h methotrexate concentration. Teniposide systemic clearance and terminal half-life and cytarabine systemic clearance were not different between the two groups. There was no significant difference in etoposide systemic clearance when normalised to body surface area (ml min-1 m-2), however a significantly lower systemic clearance relative to body weight (ml min-1 kg-1) was observed in two infants, 0.5 to 1 year of age, vs 23 children, 3-18 years of age. Doxorubicin systemic clearance was not significantly different between the two groups when systemic clearance was expressed in ml min-1 kg-1. However, there was a trend toward a lower rate of systemic clearance in ml min-1 m-2 in infants.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge A., Aranda J. V., Neims A. H. Caffeine metabolism in the newborn. Clin Pharmacol Ther. 1979 Apr;25(4):447–453. doi: 10.1002/cpt1979254447. [DOI] [PubMed] [Google Scholar]
- Allen J. C. The effects of cancer therapy on the nervous system. J Pediatr. 1978 Dec;93(6):903–909. doi: 10.1016/s0022-3476(78)81209-8. [DOI] [PubMed] [Google Scholar]
- Aranda J. V., MacLeod S. M., Renton K. W., Eade N. R. Hepatic microsomal drug oxidation and electron transport in newborn infants. J Pediatr. 1974 Oct;85(4):534–542. doi: 10.1016/s0022-3476(74)80465-8. [DOI] [PubMed] [Google Scholar]
- Besunder J. B., Reed M. D., Blumer J. L. Principles of drug biodisposition in the neonate. A critical evaluation of the pharmacokinetic-pharmacodynamic interface (Part I). Clin Pharmacokinet. 1988 Apr;14(4):189–216. doi: 10.2165/00003088-198814040-00001. [DOI] [PubMed] [Google Scholar]
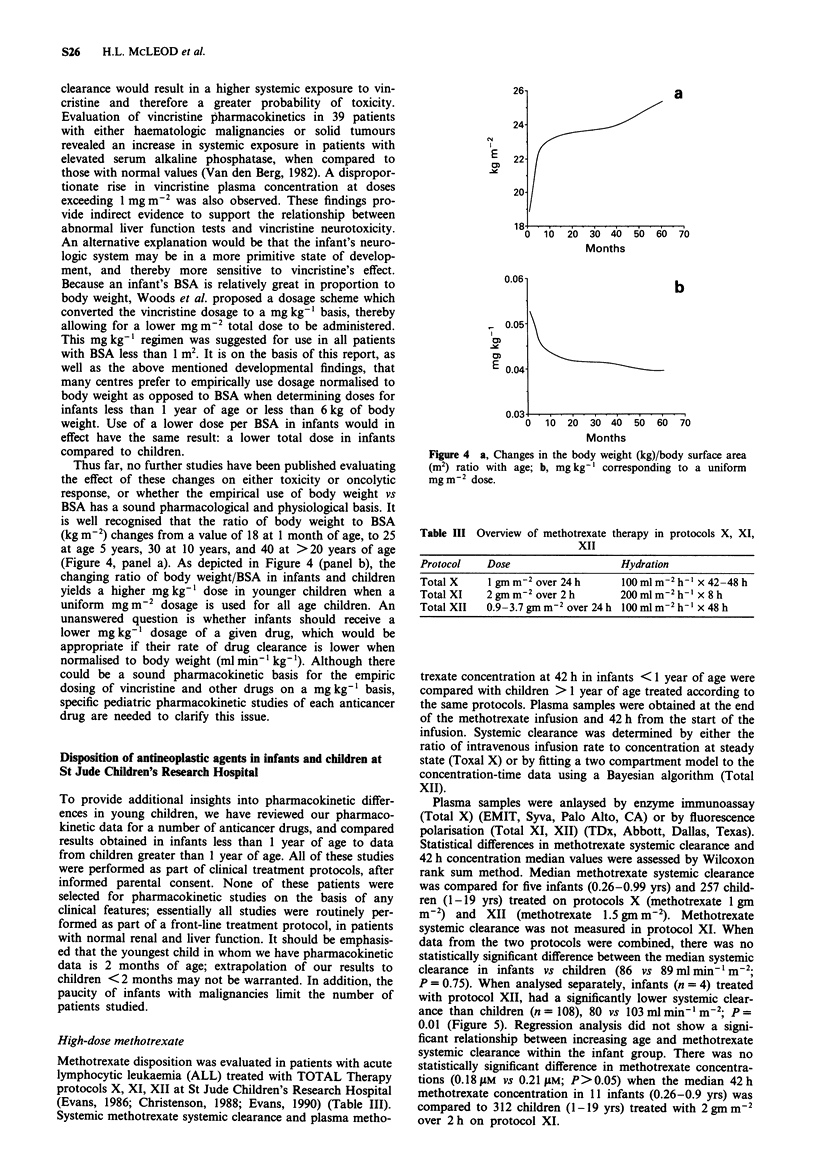
- Bleyer A. W. Clinical pharmacology of intrathecal methotrexate. II. An improved dosage regimen derived from age-related pharmacokinetics. Cancer Treat Rep. 1977 Nov;61(8):1419–1425. [PubMed] [Google Scholar]
- Christensen M. L., Rivera G. K., Crom W. R., Hancock M. L., Evans W. E. Effect of hydration on methotrexate plasma concentrations in children with acute lymphocytic leukemia. J Clin Oncol. 1988 May;6(5):797–801. doi: 10.1200/JCO.1988.6.5.797. [DOI] [PubMed] [Google Scholar]
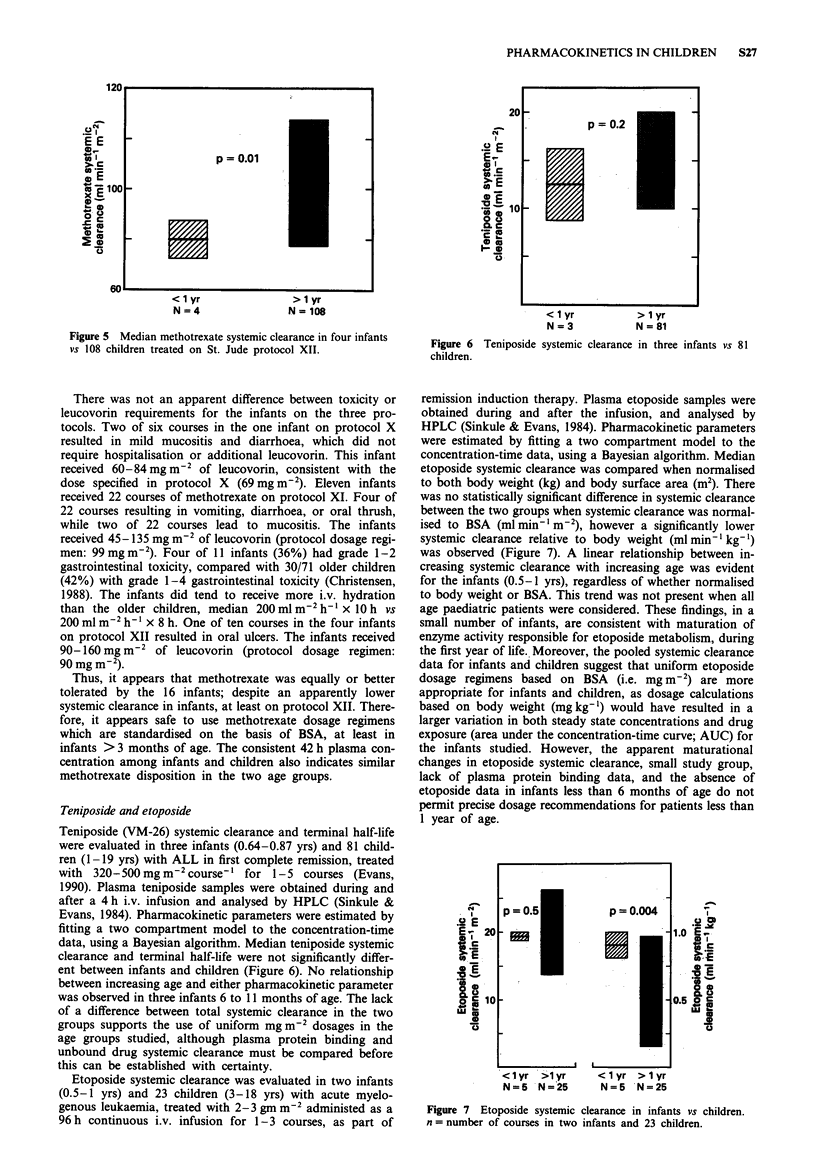
- Evans W. E., Crom W. R., Abromowitch M., Dodge R., Look A. T., Bowman W. P., George S. L., Pui C. H. Clinical pharmacodynamics of high-dose methotrexate in acute lymphocytic leukemia. Identification of a relation between concentration and effect. N Engl J Med. 1986 Feb 20;314(8):471–477. doi: 10.1056/NEJM198602203140803. [DOI] [PubMed] [Google Scholar]
- Evans W. E., Petros W. P., Relling M. V., Crom W. R., Madden T., Rodman J. H., Sunderland M. Clinical pharmacology of cancer chemotherapy in children. Pediatr Clin North Am. 1989 Oct;36(5):1199–1230. doi: 10.1016/s0031-3955(16)36765-7. [DOI] [PubMed] [Google Scholar]
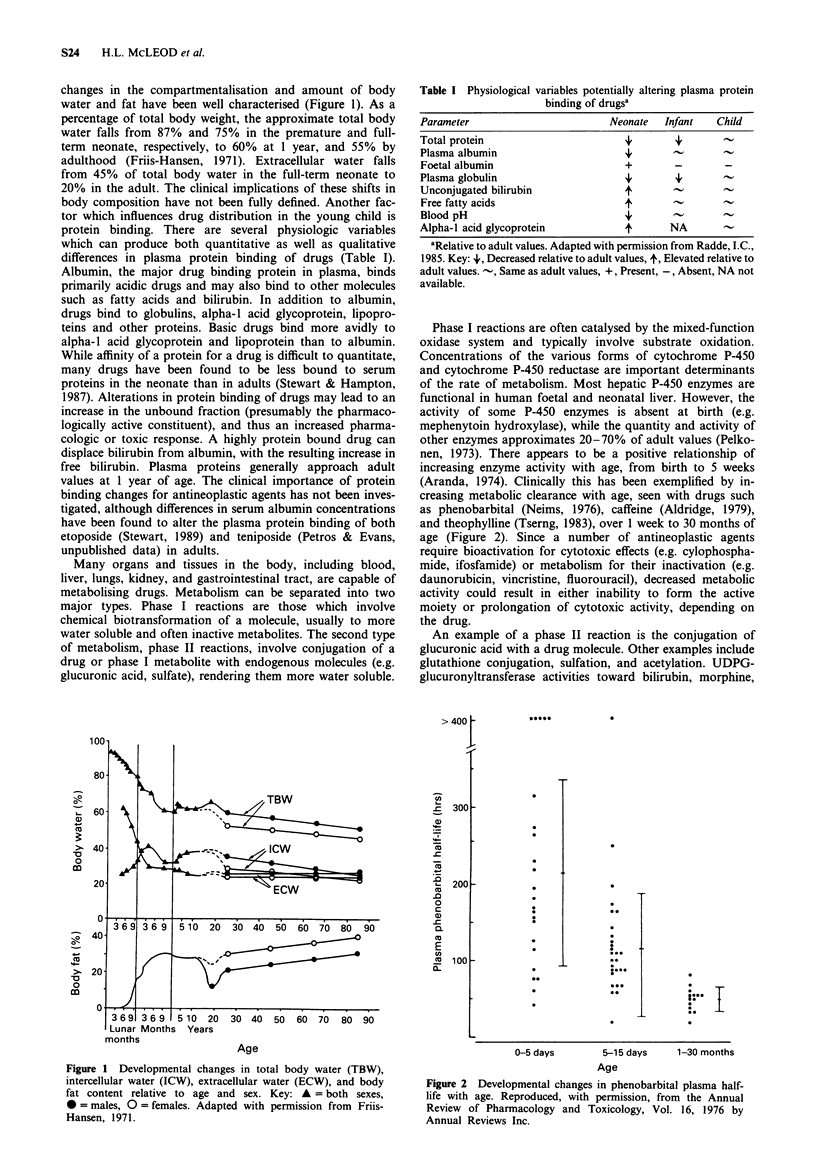
- Friis-Hansen B. Body composition during growth. In vivo measurements and biochemical data correlated to differential anatomical growth. Pediatrics. 1971 Jan;47(1 Suppl):264+–264+. [PubMed] [Google Scholar]
- Green D. M., Finklestein J. Z., Norkool P., D'Angio G. J. Severe hepatic toxicity after treatment with single-dose dactinomycin and vincristine. A report of the National Wilms' Tumor Study. Cancer. 1988 Jul 15;62(2):270–273. doi: 10.1002/1097-0142(19880715)62:2<270::aid-cncr2820620208>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Green D. M., Norkool P., Breslow N. E., Finklestein J. Z., D'Angio G. J. Severe hepatic toxicity after treatment with vincristine and dactinomycin using single-dose or divided-dose schedules: a report from the National Wilms' Tumor Study. J Clin Oncol. 1990 Sep;8(9):1525–1530. doi: 10.1200/JCO.1990.8.9.1525. [DOI] [PubMed] [Google Scholar]
- Heimann G. Enteral absorption and bioavailability in children in relation to age. Eur J Clin Pharmacol. 1980 Jul;18(1):43–50. doi: 10.1007/BF00561477. [DOI] [PubMed] [Google Scholar]
- Jones B., Breslow N. E., Takashima J. Toxic deaths in the Second National Wilms' Tumor Study. J Clin Oncol. 1984 Sep;2(9):1028–1033. doi: 10.1200/JCO.1984.2.9.1028. [DOI] [PubMed] [Google Scholar]
- Kearns G. L., Reed M. D. Clinical pharmacokinetics in infants and children. A reappraisal. Clin Pharmacokinet. 1989;17 (Suppl 1):29–67. doi: 10.2165/00003088-198900171-00005. [DOI] [PubMed] [Google Scholar]
- Leakey J. E., Hume R., Burchell B. Development of multiple activities of UDP-glucuronyltransferase in human liver. Biochem J. 1987 May 1;243(3):859–861. doi: 10.1042/bj2430859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebenthal E., Lee P. C., Heitlinger L. A. Impact of development of the gastrointestinal tract on infant feeding. J Pediatr. 1983 Jan;102(1):1–9. doi: 10.1016/s0022-3476(83)80276-5. [DOI] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S. Are children with lymphoblastic leukaemia given enough 6-mercaptopurine? Lancet. 1987 Oct 3;2(8562):785–787. doi: 10.1016/s0140-6736(87)92511-6. [DOI] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S., Van Loon J., Weinshilboum R. M. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990 Jul 28;336(8709):225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J Clin Oncol. 1989 Dec;7(12):1816–1823. doi: 10.1200/JCO.1989.7.12.1816. [DOI] [PubMed] [Google Scholar]
- Levy G., Khanna N. N., Soda D. M., Tsuzuki O., Stern L. Pharmacokinetics of acetaminophen in the human neonate: formation of acetaminophen glucuronide and sulfate in relation to plasma bilirubin concentration and D-glucaric acid excretion. Pediatrics. 1975 Jun;55(6):818–825. [PubMed] [Google Scholar]
- Morselli P. L., Franco-Morselli R., Bossi L. Clinical pharmacokinetics in newborns and infants. Age-related differences and therapeutic implications. Clin Pharmacokinet. 1980 Nov-Dec;5(6):485–527. doi: 10.2165/00003088-198005060-00001. [DOI] [PubMed] [Google Scholar]
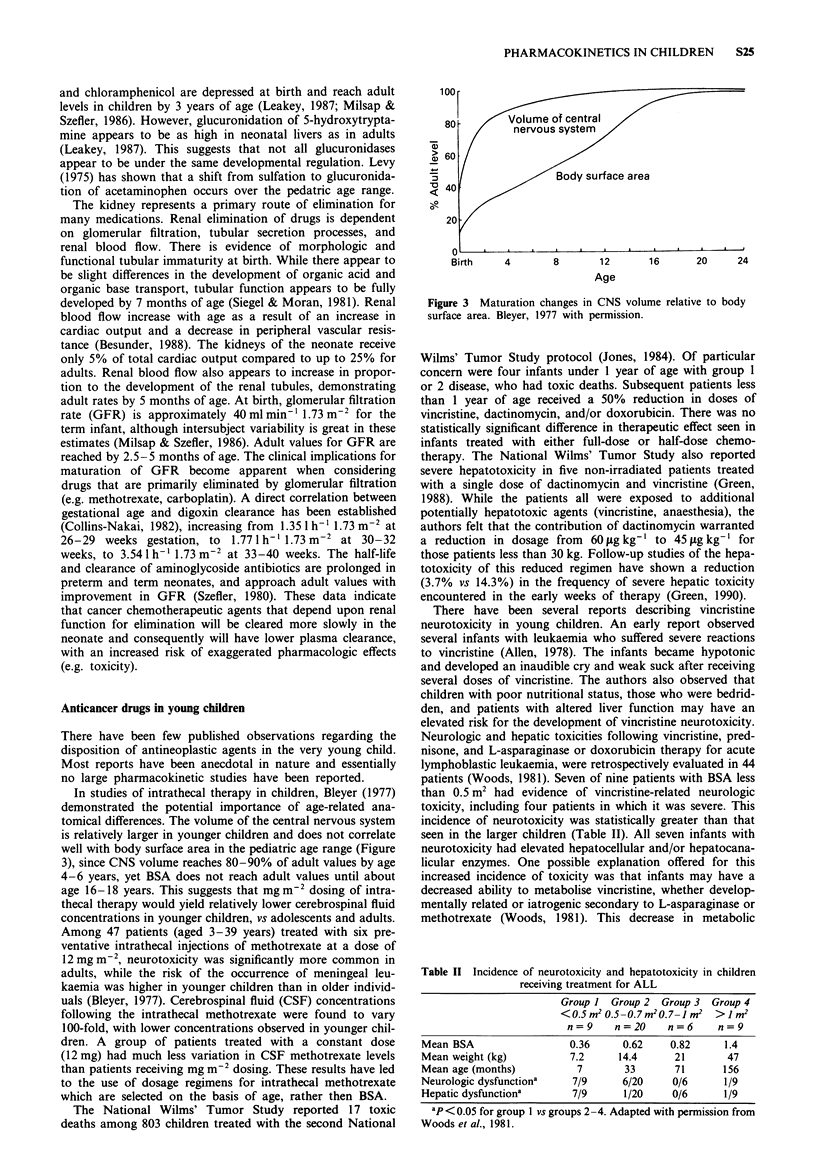
- Neims A. H., Warner M., Loughnan P. M., Aranda J. V. Developmental aspects of the hepatic cytochrome P450 monooxygenase system. Annu Rev Pharmacol Toxicol. 1976;16:427–445. doi: 10.1146/annurev.pa.16.040176.002235. [DOI] [PubMed] [Google Scholar]
- Pelkonen O., Kaltiala E. H., Larmi T. K., Kärki N. T. Comparison of activities of drug-metabolizing enzymes in human fetal and adult livers. Clin Pharmacol Ther. 1973 Sep-Oct;14(5):840–846. doi: 10.1002/cpt1973145840. [DOI] [PubMed] [Google Scholar]
- Schmiegelow K., Bruunshuus I. 6-Thioguanine nucleotide accumulation in red blood cells during maintenance chemotherapy for childhood acute lymphoblastic leukemia, and its relation to leukopenia. Cancer Chemother Pharmacol. 1990;26(4):288–292. doi: 10.1007/BF02897232. [DOI] [PubMed] [Google Scholar]
- Siegel S. E., Moran R. G. Problems in the chemotherapy of cancer in the neonate. Am J Pediatr Hematol Oncol. 1981 Fall;3(3):287–296. [PubMed] [Google Scholar]
- Sinkule J. A., Evans W. E. High-performance liquid chromatographic analysis of the semisynthetic epipodophyllotoxins teniposide and etoposide using electrochemical detection. J Pharm Sci. 1984 Feb;73(2):164–168. doi: 10.1002/jps.2600730207. [DOI] [PubMed] [Google Scholar]
- Stewart C. F., Hampton E. M. Effect of maturation on drug disposition in pediatric patients. Clin Pharm. 1987 Jul;6(7):548–564. [PubMed] [Google Scholar]
- Stewart C. F., Pieper J. A., Arbuck S. G., Evans W. E. Altered protein binding of etoposide in patients with cancer. Clin Pharmacol Ther. 1989 Jan;45(1):49–55. doi: 10.1038/clpt.1989.8. [DOI] [PubMed] [Google Scholar]
- Szefler S. J., Wynn R. J., Clarke D. F., Buckwald S., Shen D., Schentag J. J. Relationship of gentamicin serum concentrations to gestational age in preterm and term neonates. J Pediatr. 1980 Aug;97(2):312–315. doi: 10.1016/s0022-3476(80)80506-3. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y., Takieddine F. N., King K. C. Developmental aspects of theophylline metabolism in premature infants. Clin Pharmacol Ther. 1983 Apr;33(4):522–528. doi: 10.1038/clpt.1983.71. [DOI] [PubMed] [Google Scholar]
- Van den Berg H. W., Desai Z. R., Wilson R., Kennedy G., Bridges J. M., Shanks R. G. The pharmacokinetics of vincristine in man: reduced drug clearance associated with raised serum alkaline phosphatase and dose-limited elimination. Cancer Chemother Pharmacol. 1982;8(2):215–219. doi: 10.1007/BF00255487. [DOI] [PubMed] [Google Scholar]
- Woods W. G., O'Leary M., Nesbit M. E. Life-threatening neuropathy and hepatotoxicity in infants during induction therapy for acute lymphoblastic leukemia. J Pediatr. 1981 Apr;98(4):642–645. doi: 10.1016/s0022-3476(81)80785-8. [DOI] [PubMed] [Google Scholar]


