Abstract
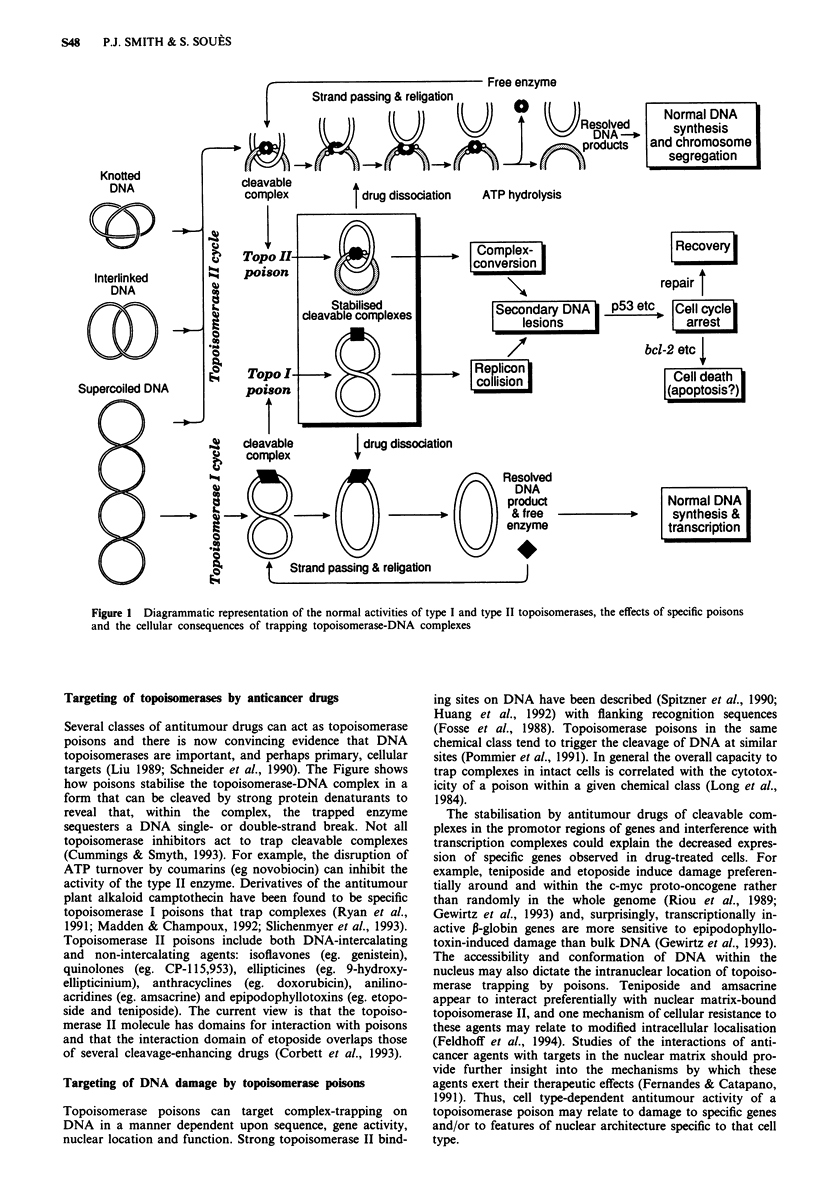
The successful use of cytotoxic agents in the clinical management of LCH depends upon the selective targeting of cells participating in the disease process. The topoisomerase 'poisons', currently used extensively in the treatment of aggressive malignancies, represent an intriguing class of cytotoxic agents exerting their cytostatic and cytotoxic effects at multiple levels according to cell type. The non-DNA intercalating topoisomerase II poison, etoposide (VP-16), is the "drug of first choice" in the treatment of LCH by cytotoxic chemotherapy. This major anticancer agent traps the nuclear enzyme DNA topoisomerase II on DNA in a sequence-specific manner, the processing of trapped complexes giving rise to a plethora of cellular effects not least the potential activation of pathways leading to cell cycle arrest and apoptosis. This short review describes the principles of topoisomerase inhibition, the multiplicity of cellular effects and the concept of cellular targeting in LCH. The successful treatment of LCH by cytotoxic chemotherapy will depend on both the identity of the target tissues and a clear view of therapeutic intent, given the potential for induction of haematological neoplasia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand R., Solary E., Jenkins J., Pommier Y. Apoptosis and its modulation in human promyelocytic HL-60 cells treated with DNA topoisomerase I and II inhibitors. Exp Cell Res. 1993 Aug;207(2):388–397. doi: 10.1006/excr.1993.1206. [DOI] [PubMed] [Google Scholar]
- Chow K. C., King C. K., Ross W. E. Abrogation of etoposide-mediated cytotoxicity by cycloheximide. Biochem Pharmacol. 1988 Mar 15;37(6):1117–1122. doi: 10.1016/0006-2952(88)90519-9. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Corbett A. H., Hong D., Osheroff N. Exploiting mechanistic differences between drug classes to define functional drug interaction domains on topoisomerase II. Evidence that several diverse DNA cleavage-enhancing agents share a common site of action on the enzyme. J Biol Chem. 1993 Jul 5;268(19):14394–14398. [PubMed] [Google Scholar]
- Dive C., Evans C. A., Whetton A. D. Induction of apoptosis--new targets for cancer chemotherapy. Semin Cancer Biol. 1992 Dec;3(6):417–427. [PubMed] [Google Scholar]
- Dombernowsky P., Nissen N. I. Schedule dependency of the antileukemic activity of the podophyllotoxin-derivative VP 16-213 (NSC-141540) in L1210 leukemia. Acta Pathol Microbiol Scand A. 1973 Sep;81(5):715–724. doi: 10.1111/j.1699-0463.1973.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Falk S. J., Smith P. J. DNA damaging and cell cycle effects of the topoisomerase I poison camptothecin in irradiated human cells. Int J Radiat Biol. 1992 Jun;61(6):749–757. doi: 10.1080/09553009214551601. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Fernandes D. J., Catapano C. V. Nuclear matrix targets for anticancer agents. Cancer Cells. 1991 Apr;3(4):134–140. [PubMed] [Google Scholar]
- Fosse P., Paoletti C., Saucier J. M. Pattern of recognition of DNA by mammalian DNA topoisomerase II. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1233–1240. doi: 10.1016/s0006-291x(88)80498-4. [DOI] [PubMed] [Google Scholar]
- Gewirtz D. A., Orr M. S., Fornari F. A., Randolph J. K., Yalowich J. C., Ritke M. K., Povirk L. F., Bunch R. T. Dissociation between bulk damage to DNA and the antiproliferative activity of teniposide (VM-26) in the MCF-7 breast tumor cell line: evidence for induction of gene-specific damage and alterations in gene expression. Cancer Res. 1993 Aug 1;53(15):3547–3554. [PubMed] [Google Scholar]
- Giaccone G., Gazdar A. F., Beck H., Zunino F., Capranico G. Multidrug sensitivity phenotype of human lung cancer cells associated with topoisomerase II expression. Cancer Res. 1992 Apr 1;52(7):1666–1674. [PubMed] [Google Scholar]
- Giovanella B. C., Stehlin J. S., Wall M. E., Wani M. C., Nicholas A. W., Liu L. F., Silber R., Potmesil M. DNA topoisomerase I--targeted chemotherapy of human colon cancer in xenografts. Science. 1989 Nov 24;246(4933):1046–1048. doi: 10.1126/science.2555920. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Isobe K., Nakashima I., Shimokata K. Higher expression of topoisomerase II in lung cancers than normal lung tissues: different expression pattern from topoisomerase I. Biochem Biophys Res Commun. 1993 Aug 31;195(1):409–414. doi: 10.1006/bbrc.1993.2058. [DOI] [PubMed] [Google Scholar]
- Heck M. M., Hittelman W. N., Earnshaw W. C. Differential expression of DNA topoisomerases I and II during the eukaryotic cell cycle. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1086–1090. doi: 10.1073/pnas.85.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. W., Juang J. K., Liu H. J. The recognition of DNA cleavage sites by porcine spleen topoisomerase II. Nucleic Acids Res. 1992 Feb 11;20(3):467–473. doi: 10.1093/nar/20.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J. R., Ayton P., Jones T., Davies S. L., Simmons D. L., Harris A. L., Sheer D., Hickson I. D. Isolation of cDNA clones encoding the beta isozyme of human DNA topoisomerase II and localisation of the gene to chromosome 3p24. Nucleic Acids Res. 1992 Nov 11;20(21):5587–5592. doi: 10.1093/nar/20.21.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan C. C., Hwang J. L., Liu A. A., Whang-Peng J., Knutsen T., Huebner K., Croce C. M., Zhang H., Wang J. C., Liu L. F. Human DNA topoisomerase I is encoded by a single-copy gene that maps to chromosome region 20q12-13.2. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8910–8913. doi: 10.1073/pnas.85.23.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989 Nov 1;49(21):5870–5878. [PubMed] [Google Scholar]
- Kroll D. J., Rowe T. C. Phosphorylation of DNA topoisomerase II in a human tumor cell line. J Biol Chem. 1991 Apr 25;266(12):7957–7961. [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lee M. P., Brown S. D., Chen A., Hsieh T. S. DNA topoisomerase I is essential in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6656–6660. doi: 10.1073/pnas.90.14.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Lock R. B., Ross W. E. Possible role for p34cdc2 kinase in etoposide-induced cell death of Chinese hamster ovary cells. Cancer Res. 1990 Jun 15;50(12):3767–3771. [PubMed] [Google Scholar]
- Long B. H., Musial S. T., Brattain M. G. Comparison of cytotoxicity and DNA breakage activity of congeners of podophyllotoxin including VP16-213 and VM26: a quantitative structure-activity relationship. Biochemistry. 1984 Mar 13;23(6):1183–1188. doi: 10.1021/bi00301a024. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Madden K. R., Champoux J. J. Overexpression of human topoisomerase I in baby hamster kidney cells: hypersensitivity of clonal isolates to camptothecin. Cancer Res. 1992 Feb 1;52(3):525–532. [PubMed] [Google Scholar]
- Merino A., Madden K. R., Lane W. S., Champoux J. J., Reinberg D. DNA topoisomerase I is involved in both repression and activation of transcription. Nature. 1993 Sep 16;365(6443):227–232. doi: 10.1038/365227a0. [DOI] [PubMed] [Google Scholar]
- Negri C., Chiesa R., Cerino A., Bestagno M., Sala C., Zini N., Maraldi N. M., Astaldi Ricotti G. C. Monoclonal antibodies to human DNA topoisomerase I and the two isoforms of DNA topoisomerase II: 170- and 180-kDa isozymes. Exp Cell Res. 1992 Jun;200(2):452–459. doi: 10.1016/0014-4827(92)90195-e. [DOI] [PubMed] [Google Scholar]
- Pedersen-Bjergaard J., Sigsgaard T. C., Nielsen D., Gjedde S. B., Philip P., Hansen M., Larsen S. O., Rørth M., Mouridsen H., Dombernowsky P. Acute monocytic or myelomonocytic leukemia with balanced chromosome translocations to band 11q23 after therapy with 4-epi-doxorubicin and cisplatin or cyclophosphamide for breast cancer. J Clin Oncol. 1992 Sep;10(9):1444–1451. doi: 10.1200/JCO.1992.10.9.1444. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Capranico G., Orr A., Kohn K. W. Local base sequence preferences for DNA cleavage by mammalian topoisomerase II in the presence of amsacrine or teniposide. Nucleic Acids Res. 1991 Nov 11;19(21):5973–5980. doi: 10.1093/nar/19.21.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou J. F., Lefevre D., Riou G. Stimulation of the topoisomerase II induced DNA cleavage sites in the c-myc protooncogene by antitumor drugs is associated with gene expression. Biochemistry. 1989 Nov 14;28(23):9104–9110. doi: 10.1021/bi00449a022. [DOI] [PubMed] [Google Scholar]
- Ryan A. J., Squires S., Strutt H. L., Johnson R. T. Camptothecin cytotoxicity in mammalian cells is associated with the induction of persistent double strand breaks in replicating DNA. Nucleic Acids Res. 1991 Jun 25;19(12):3295–3300. doi: 10.1093/nar/19.12.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo M., Enomoto T., Hanaoka F., Ui M. Purification and characterization of type II DNA topoisomerase from mouse FM3A cells: phosphorylation of topoisomerase II and modification of its activity. Biochemistry. 1990 Jan 16;29(2):583–590. doi: 10.1021/bi00454a036. [DOI] [PubMed] [Google Scholar]
- Saijo M., Ui M., Enomoto T. Growth state and cell cycle dependent phosphorylation of DNA topoisomerase II in Swiss 3T3 cells. Biochemistry. 1992 Jan 21;31(2):359–363. doi: 10.1021/bi00117a007. [DOI] [PubMed] [Google Scholar]
- Schneider E., Hsiang Y. H., Liu L. F. DNA topoisomerases as anticancer drug targets. Adv Pharmacol. 1990;21:149–183. doi: 10.1016/s1054-3589(08)60342-7. [DOI] [PubMed] [Google Scholar]
- Schneider E., Lawson P. A., Ralph R. K. Inhibition of protein synthesis reduces the cytotoxicity of 4'-(9-acridinylamino)methanesulfon-m-anisidide without affecting DNA breakage and DNA topoisomerase II in a murine mastocytoma cell line. Biochem Pharmacol. 1989 Jan 15;38(2):263–269. doi: 10.1016/0006-2952(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Slichenmyer W. J., Rowinsky E. K., Donehower R. C., Kaufmann S. H. The current status of camptothecin analogues as antitumor agents. J Natl Cancer Inst. 1993 Feb 17;85(4):271–291. doi: 10.1093/jnci/85.4.271. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Makinson T. A. Cellular consequences of overproduction of DNA topoisomerase II in an ataxia-telangiectasia cell line. Cancer Res. 1989 Mar 1;49(5):1118–1124. [PubMed] [Google Scholar]
- Spitzner J. R., Chung I. K., Muller M. T. Eukaryotic topoisomerase II preferentially cleaves alternating purine-pyrimidine repeats. Nucleic Acids Res. 1990 Jan 11;18(1):1–11. doi: 10.1093/nar/18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., Dorman T. E., Falls K. M., Chung T. D., Mirabelli C. K., Crooke S. T., Mao J. Topoisomerase II alpha and topoisomerase II beta genes: characterization and mapping to human chromosomes 17 and 3, respectively. Cancer Res. 1992 Jan 1;52(1):231–234. [PubMed] [Google Scholar]
- Tsai-Pflugfelder M., Liu L. F., Liu A. A., Tewey K. M., Whang-Peng J., Knutsen T., Huebner K., Croce C. M., Wang J. C. Cloning and sequencing of cDNA encoding human DNA topoisomerase II and localization of the gene to chromosome region 17q21-22. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7177–7181. doi: 10.1073/pnas.85.19.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura K., Hirose S. Possible role of DNA topoisomerase II on transcription of the homeobox gene Hox-2.1 in F9 embryonal carcinoma cells. Nucleic Acids Res. 1991 Nov 25;19(22):6087–6092. doi: 10.1093/nar/19.22.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Webb C. D., Latham M. D., Lock R. B., Sullivan D. M. Attenuated topoisomerase II content directly correlates with a low level of drug resistance in a Chinese hamster ovary cell line. Cancer Res. 1991 Dec 15;51(24):6543–6549. [PubMed] [Google Scholar]
- Whitlock J. A., Greer J. P., Lukens J. N. Epipodophyllotoxin-related leukemia. Identification of a new subset of secondary leukemia. Cancer. 1991 Aug 1;68(3):600–604. doi: 10.1002/1097-0142(19910801)68:3<600::aid-cncr2820680326>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Woessner R. D., Chung T. D., Hofmann G. A., Mattern M. R., Mirabelli C. K., Drake F. H., Johnson R. K. Differences between normal and ras-transformed NIH-3T3 cells in expression of the 170kD and 180kD forms of topoisomerase II. Cancer Res. 1990 May 15;50(10):2901–2908. [PubMed] [Google Scholar]
- Wolff S. N., Grosh W. W., Prater K., Hande K. R. In vitro pharmacodynamic evaluation of VP-16-213 and implications for chemotherapy. Cancer Chemother Pharmacol. 1987;19(3):246–249. doi: 10.1007/BF00252980. [DOI] [PubMed] [Google Scholar]
- Wood E. R., Earnshaw W. C. Mitotic chromatin condensation in vitro using somatic cell extracts and nuclei with variable levels of endogenous topoisomerase II. J Cell Biol. 1990 Dec;111(6 Pt 2):2839–2850. doi: 10.1083/jcb.111.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini N., Martelli A. M., Sabatelli P., Santi S., Negri C., Astaldi Ricotti G. C., Maraldi N. M. The 180-kDa isoform of topoisomerase II is localized in the nucleolus and belongs to the structural elements of the nucleolar remnant. Exp Cell Res. 1992 Jun;200(2):460–466. doi: 10.1016/0014-4827(92)90196-f. [DOI] [PubMed] [Google Scholar]


