Abstract
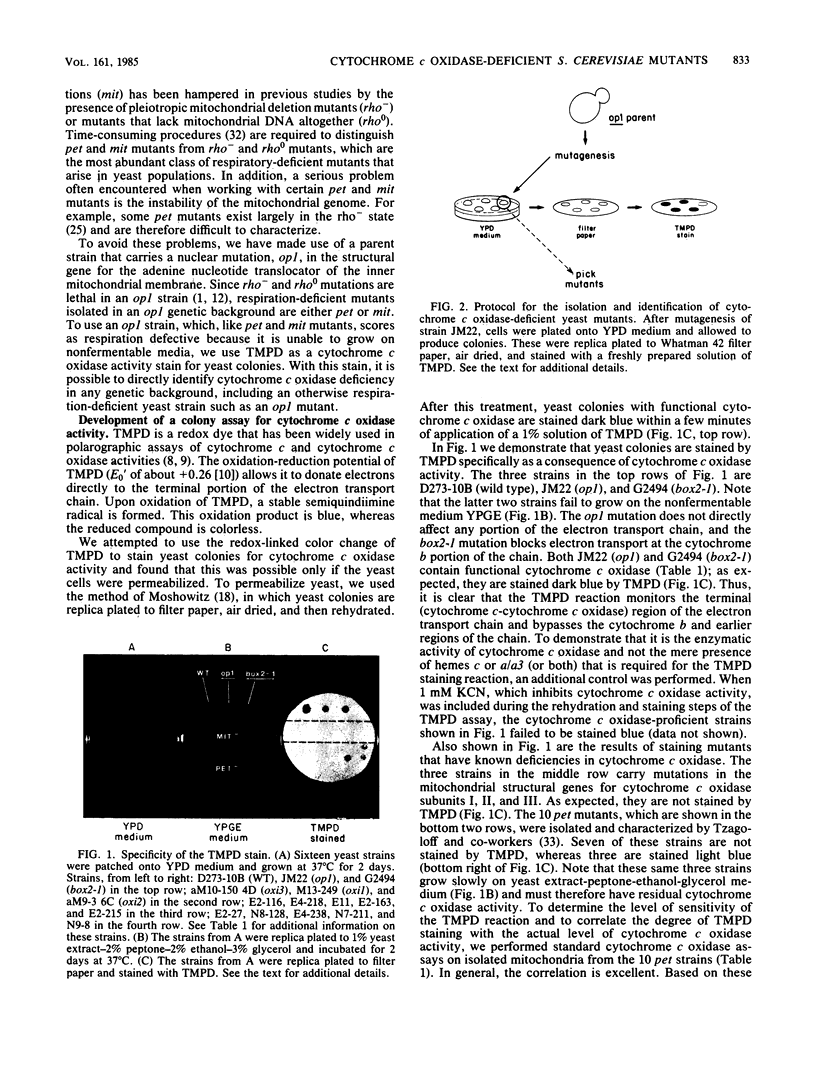
We describe here a new method for the specific isolation of cytochrome c oxidase-deficient mutants of Saccharomyces cerevisiae. One unique feature of the method is the use of tetramethyl-p-phenylenediamine as a cytochrome c oxidase activity stain for yeast colonies. The staining of yeast colonies by tetramethyl-p-phenylenediamine is dependent upon a functional cytochrome c oxidase and is unaffected by other lesions in respiration. Since the tetramethyl-p-phenylenediamine colony staining reaction is rapid and simple, it greatly facilitates both the identification and characterization of cytochrome c oxidase-deficient mutants. Another feature of the method, which is made possible by the tetramethyl-p-phenylenediamine colony stain, is the use of an op1 parent strain for the isolation of nuclear pet or mitochondrial mit mutants in specific protein-coding genes. A parent strain that carries this marker selects against rho0 or rho- classes of pleiotropic respiratory-deficient mutants, since these are lethal in op1 strains. We have used this method to isolate 123 independently derived cytochrome c oxidase-deficient pet mutants and 300 independently derived mit mutants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. C., Mattoon J. R., Hawthorne D. C., Sherman F. Genetic modification of energy-conserving systems in yeast mitochondria. Proc Natl Acad Sci U S A. 1968 May;60(1):186–193. doi: 10.1073/pnas.60.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. Assembly of the mitochondrial membrane system. Physical map of the Oxi3 locus of yeast mitochondrial DNA. J Biol Chem. 1980 Dec 25;255(24):11922–11926. [PubMed] [Google Scholar]
- Cabral F., Schatz G. Identification of cytochrome c oxidase subunits in nuclear yeast mutants lacking the functional enzyme. J Biol Chem. 1978 Jun 25;253(12):4396–4401. [PubMed] [Google Scholar]
- Coruzzi G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence of subunit 2 of yeast cytochrome oxidase. J Biol Chem. 1979 Sep 25;254(18):9324–9330. [PubMed] [Google Scholar]
- Cumsky M. G., McEwen J. E., Ko C., Poyton R. O. Nuclear genes for mitochondrial proteins. Identification and isolation of a structural gene for subunit V of yeast cytochrome c oxidase. J Biol Chem. 1983 Nov 25;258(22):13418–13421. [PubMed] [Google Scholar]
- Ebner E., Mennucci L., Schatz G. Mitochondrial assembly in respiration-deficient mutants of Saccharomyces cerevisiae. I. Effect of nuclear mutations on mitochondrial protein synthesis. J Biol Chem. 1973 Aug 10;248(15):5360–5368. [PubMed] [Google Scholar]
- Faye G., Simon M. Analysis of a yeast nuclear gene involved in the maturation of mitochondrial pre-messenger RNA of the cytochrome oxidase subunit I. Cell. 1983 Jan;32(1):77–87. doi: 10.1016/0092-8674(83)90498-1. [DOI] [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Definition of cytochrome c binding domains by chemical modification. III. Kinetics of reaction of carboxydinitrophenyl cytochromes c with cytochrome c oxidase. J Biol Chem. 1978 Jan 10;253(1):149–159. [PubMed] [Google Scholar]
- Hill B. C., Nicholls P. Reduction and activity of cytochrome c in the cytochrome c-cytochrome aa3 complex. Biochem J. 1980 Jun 1;187(3):809–818. doi: 10.1042/bj1870809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS E. E. Phosphorylation coupled to electron transport initiated by substituted phenylenediamines. Biochem Biophys Res Commun. 1960 Nov;3:536–539. doi: 10.1016/0006-291x(60)90170-4. [DOI] [PubMed] [Google Scholar]
- Kovácová V., Irmlerová J., Kovác L. Oxidative phosphorylatiion in yeast. IV. Combination of a nuclear mutation affecting oxidative phosphorylation with cytoplasmic mutation to respiratory deficiency. Biochim Biophys Acta. 1968 Aug 20;162(2):157–163. doi: 10.1016/0005-2728(68)90097-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mason T. L., Schatz G. Cytochrome c oxidase from bakers' yeast. II. Site of translation of the protein components. J Biol Chem. 1973 Feb 25;248(4):1355–1360. [PubMed] [Google Scholar]
- McEwen J. E., Cumsky M. G., Ko C., Power S. D., Poyton R. O. Mitochondrial membrane biogenesis: characterization and use of pet mutants to clone the nuclear gene coding for subunit V of yeast cytochrome c oxidase. J Cell Biochem. 1984;24(3):229–242. doi: 10.1002/jcb.240240305. [DOI] [PubMed] [Google Scholar]
- McKee E. E., McEwen J. E., Poyton R. O. Mitochondrial gene expression in saccharomyces cerevisiae. II. Fidelity of translation in isolated mitochondria from wild type and respiratory-deficient mutant cells. J Biol Chem. 1984 Jul 25;259(14):9332–9338. [PubMed] [Google Scholar]
- Mowshowitz D. B. Permeabilization of yeast for enzyme assays: an extremely simple method for small samples. Anal Biochem. 1976 Jan;70(1):94–99. doi: 10.1016/s0003-2697(76)80051-6. [DOI] [PubMed] [Google Scholar]
- Mustafa M. G., King T. E. Wurster's blue mediated oxidation of NADH and phosphorylation in mitochondria. Arch Biochem Biophys. 1967 Nov;122(2):501–508. doi: 10.1016/0003-9861(67)90225-1. [DOI] [PubMed] [Google Scholar]
- Müller P. P., Reif M. K., Zonghou S., Sengstag C., Mason T. L., Fox T. D. A nuclear mutation that post-transcriptionally blocks accumulation of a yeast mitochondrial gene product can be suppressed by a mitochondrial gene rearrangement. J Mol Biol. 1984 Jun 5;175(4):431–452. doi: 10.1016/0022-2836(84)90178-5. [DOI] [PubMed] [Google Scholar]
- Power S. D., Lochrie M. A., Sevarino K. A., Patterson T. E., Poyton R. O. The nuclear-coded subunits of yeast cytochrome c oxidase. I. Fractionation of the holoenzyme into chemically pure polypeptides and the identification of two new subunits using solvent extraction and reversed phase high performance liquid chromatography. J Biol Chem. 1984 May 25;259(10):6564–6570. [PubMed] [Google Scholar]
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. III. Physical characterization of isolated subunits and chemical evidence for two different classes of polypeptides. J Biol Chem. 1975 Jan 25;250(2):752–761. [PubMed] [Google Scholar]
- Pratje E., Mannhaupt G., Michaelis G., Beyreuther K. A nuclear mutation prevents processing of a mitochondrially encoded membrane protein in Saccharomyces cerevisiae. EMBO J. 1983;2(7):1049–1054. doi: 10.1002/j.1460-2075.1983.tb01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putrament A., Baranowska H., Ejchart A., Jachymczyk W. Manganese mutagenesis in yeast. VI. Mn2+ uptake, mitDNA replication and ER induction: comparison with other divalent cations. Mol Gen Genet. 1977 Feb 28;151(1):69–76. doi: 10.1007/BF00446914. [DOI] [PubMed] [Google Scholar]
- SHERMAN F. MUTANTS OF YEAST DEFICIENT IN CYTOCHROME C. Genetics. 1964 Jan;49:39–48. doi: 10.1093/genetics/49.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERMAN F. Respiration-deficient mutants of yeast. I. Genetics. Genetics. 1963 Mar;48:375–385. doi: 10.1093/genetics/48.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Faye G. Steps in processing of the mitochondrial cytochrome oxidase subunit I pre-mRNA affected by a nuclear mutation in yeast. Proc Natl Acad Sci U S A. 1984 Jan;81(1):8–12. doi: 10.1073/pnas.81.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalenfeld B. E., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequence of the oxi 2 gene of yeast mitochondrial DNA. J Biol Chem. 1980 Jul 10;255(13):6173–6180. [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Needleman R. B. Assembly of the mitochondrial membrane system. Characterization of nuclear mutants of Saccharomyces cerevisiae with defects in mitochondrial ATPase and respiratory enzymes. J Biol Chem. 1975 Oct 25;250(20):8228–8235. [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Needleman R. B. Assembly of the mitochondrial membrane system: isolation of nuclear and cytoplasmic mutants of Saccharomyces cerevisiae with specific defects in mitochondrial functions. J Bacteriol. 1975 Jun;122(3):826–831. doi: 10.1128/jb.122.3.826-831.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]




