Abstract
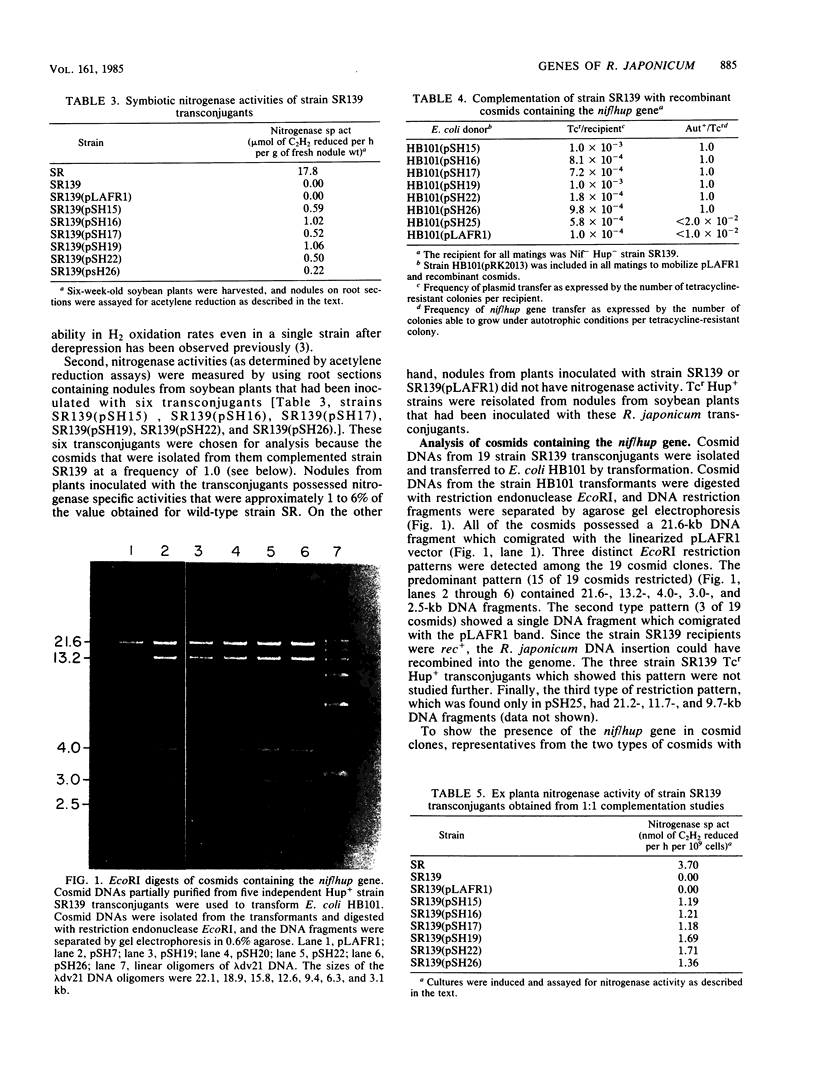
Recombinant cosmids containing a Rhizobium japonicum gene involved in both hydrogenase (Hup) and nitrogenase (Nif) activities were isolated. An R. japonicum gene bank utilizing broad-host-range cosmid pLAFR1 was conjugated into Hup- Nif- R. japonicum strain SR139. Transconjugants containing the nif/hup cosmid were identified by their resistance to tetracycline (Tcr) and ability to grow chemoautotrophically (Aut+) with hydrogen. All Tcr Aut+ transconjugants possessed high levels of H2 uptake activity, as determined amperometrically. Moreover, all Hup+ transconjugants tested possessed the ability to reduce acetylene (Nif+) in soybean nodules. Cosmid DNAs from 19 Hup+ transconjugants were transferred to Escherichia coli by transformation. When the cosmids were restricted with EcoRI, 15 of the 19 cosmids had a restriction pattern with 13.2-, 4.0-, 3.0-, and 2.5-kilobase DNA fragments. Six E. coli transformants containing the nif/hup cosmids were conjugated with strain SR139. All strain SR139 transconjugants were Hup+ Nif+. Moreover, one nif/hup cosmid was transferred to 15 other R. japonicum Hup- mutants. Hup+ transconjugants of six of the Hup- mutants appeared at a frequency of 1.0, whereas the transconjugants of the other nine mutants remained Hup-. These results indicate that the nif/hup gene cosmids contain a gene involved in both nitrogenase and hydrogenase activities and at least one and perhaps other hup genes which are exclusively involved in H2 uptake activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal A. K., Keister D. L. Physiology of Ex Planta Nitrogenase Activity in Rhizobium japonicum. Appl Environ Microbiol. 1983 May;45(5):1592–1601. doi: 10.1128/aem.45.5.1592-1601.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cantrell M. A., Haugland R. A., Evans H. J. Construction of a Rhizobium japonicum gene bank and use in isolation of a hydrogen uptake gene. Proc Natl Acad Sci U S A. 1983 Jan;80(1):181–185. doi: 10.1073/pnas.80.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Fuhrmann M., Hennecke H. Rhizobium japonicum nitrogenase Fe protein gene (nifH). J Bacteriol. 1984 Jun;158(3):1005–1011. doi: 10.1128/jb.158.3.1005-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus F. J., Maier R. J., Evans H. J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R. A., Cantrell M. A., Beaty J. S., Hanus F. J., Russell S. A., Evans H. J. Characterization of Rhizobium japonicum hydrogen uptake genes. J Bacteriol. 1984 Sep;159(3):1006–1012. doi: 10.1128/jb.159.3.1006-1012.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom S. S., Uratsu S. L., Hoang F. Transposon Tn5-induced mutagenesis of Rhizobium japonicum yielding a wide variety of mutants. J Bacteriol. 1984 Jul;159(1):335–340. doi: 10.1128/jb.159.1.335-340.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepo J. E., Hanus F. J., Evans H. J. Chemoautotrophic growth of hydrogen-uptake-positive strains of Rhizobium japonicum. J Bacteriol. 1980 Feb;141(2):664–670. doi: 10.1128/jb.141.2.664-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepo J. E., Hickok R. E., Cantrell M. A., Russell S. A., Evans H. J. Revertible hydrogen uptake-deficient mutants of Rhizobium japonicum. J Bacteriol. 1981 May;146(2):614–620. doi: 10.1128/jb.146.2.614-620.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. T., Shanmugam K. T. Regulation of hydrogen utilisation in Rhizobium japonicum by cyclic AMP. Biochim Biophys Acta. 1979 May 16;584(3):479–492. doi: 10.1016/0304-4165(79)90121-1. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Mutant Strains of Rhizobium japonicum with Increased Ability to Fix Nitrogen for Soybean. Science. 1978 Aug 4;201(4354):448–450. doi: 10.1126/science.201.4354.448. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Hanus F. J., Evans H. J. Regulation of hydrogenase in Rhizobium japonicum. J Bacteriol. 1979 Feb;137(2):825–829. doi: 10.1128/jb.137.2.825-829.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Merberg D. M. Rhizobium japonicum mutants that are hypersensitive to repression of H2 uptake by oxygen. J Bacteriol. 1982 Apr;150(1):161–167. doi: 10.1128/jb.150.1.161-167.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Mutaftschiev S. Reconstitution of H2 oxidation activity from H2 uptake-negative mutants of Rhizobium japonicum bacteroids. J Biol Chem. 1982 Feb 25;257(4):2092–2096. [PubMed] [Google Scholar]
- Maier R. J. Rhizobium japonicum mutant strains unable to grow chemoautotrophically with H2. J Bacteriol. 1981 Jan;145(1):533–540. doi: 10.1128/jb.145.1.533-540.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshiri F., Stults L., Novak P., Maier R. J. Nif- Hup- mutants of Rhizobium japonicum. J Bacteriol. 1983 Aug;155(2):926–929. doi: 10.1128/jb.155.2.926-929.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Evans H. J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stults L. W., O'Hara E. B., Maier R. J. Nickel is a component of hydrogenase in Rhizobium japonicum. J Bacteriol. 1984 Jul;159(1):153–158. doi: 10.1128/jb.159.1.153-158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Healey F. P., Myers J. Amperometric measurement of hydrogen evolution in chlamydomonas. Plant Physiol. 1971 Jul;48(1):108–110. doi: 10.1104/pp.48.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]




