Abstract
In the present study, we have used the two-electrode voltage-clamp and patch-clamp techniques to study the effects of forskolin and cAMP on the ROMK1 channels, which are believed to be the native K+ secretory channels in the kidney. Addition of 1 μM forskolin or 100 μM 8-bromo-cAMP, within 10 min, has no significant effect on the current of ROMK1 channels expressed in Xenopus oocytes. In contrast, application of 1 μM forskolin, within 3 min, significantly increased whole-cell K+ current by 35%, when ROMK1 channels were coexpressed with the A kinase anchoring protein AKAP79, which was cloned from neuronal tissue. Two lines of evidence indicate that the effect of forskolin is mediated by a cAMP-dependent pathway: (i) Addition of 100 μM 8-bromo-cAMP mimics the effect of forskolin and (ii) the effect of forskolin and cAMP is not additive. That AKAP is required for the effect of cAMP is further supported by experiments in which addition of ATP (100 μM) and cAMP (100 μM) restored the activity of run-down ROMK1 channels in inside-out patches in oocytes that coexpressed ROMK1 and AKAP79 but not in those that expressed ROMK1 alone. Moreover, when we used RII, the regulatory subunit of type II protein kinase A, in an overlay assay, we identified a RII-binding protein in membranes obtained from the kidney cortex but not in membranes from oocytes. This suggests that the insensitivity of ROMK1 channels to forskolin and cAMP is due to the absence of AKAPs. We conclude that AKAP may be a critical component that mediates the effect of protein kinase A on the ROMK channels in the kidney.
Keywords: collecting duct/thick ascending limb/kidney/K+ secretion
We have previously demonstrated that stimulation of protein kinase A (PKA) activates the ROMK-like K+ secretory channel in the thick ascending limb (TAL) and cortical collecting duct (CCD) (1). The ROMK channel is an important member of the family of inwardly rectifying K+ channels with two transmembrane segments (2). Several lines of evidence indicate that the ROMK channels are closely related to the renal low-conductance ATP-sensitive K+ channels located in the TAL and in the CCD (1). (i) In situ hybridization has shown the presence of mRNA encoding ROMK channels in the CCD and the TAL. Immunocytochemical studies have further revealed the presence of ROMK channels in the apical membrane where renal ATP-sensitive K+ channels have been identified (3, 4). (ii) The conductance (30–40 pS) and kinetics of ROMK channels expressed in oocytes are identical to those of the native low-conductance ATP-sensitive K+ channels in the CCD and the TAL (5, 6). (iii) Both the low-conductance ATP-sensitive K+ channel and the ROMK channel are stimulated by PKA-induced phosphorylation (7).
Recently, it has been suggested that the A kinase anchoring protein (AKAP) is required for mediating PKA-induced phosphorylation of cellular proteins (8–10). AKAPs are involved in the localization of the type II isoform of PKA in a variety of cell membranes (9). More than 70% of the type II of PKA is bound to the cell membrane (11). Several studies have recently shown that AKAPs are required for the stimulatory effect of PKA on L-type Ca2+ channels (12, 13) and on α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-gated ion channels (14). In the present study, we explore the involvement of AKAPs in mediating the PKA-induced activation of ROMK channels.
METHODS
Preparation of Xenopus Oocytes.
Xenopus laevis females were obtained from Nasco (Fort Atkinson, WI). The method for obtaining oocytes has been described (15). The follicular layer of the oocytes was separated under a dissecting microscope with watch-maker’s forceps. The oocytes were incubated overnight at 19°C in an incubation medium containing 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM Hepes⋅NaOH (pH 7.4), 2.5 mM pyruvate, and 50 μg/ml gentamicin (ND96). Viable oocytes were selected and microinjected with ROMK1 cRNA alone or with a mixture of ROMK1 and AKAP79 cRNAs. The cRNA encoding ROMK1 channel was prepared by in vitro transcription by subcloning the cDNA into the XhoI–BglIII multicloning site of the pSPORT vector (GIBCO/BRL). Oocytes were injected with 10 ng of ROMK1 cRNA per egg and 15 ng of AKAP79 cRNA per egg. The oocytes were incubated at 19°C in ND96 medium and experiments were performed on days 3–5 after injection.
Patch-Clamp Technique.
Patch-clamp electrodes were pulled from glass capillary tubes (Dagan, Minneapolis), with a resistance of 4–6 MΩ when filled with a pipette solution containing 150 mM KCl, 1 mM CaCl2, 1 mM MgCl2 and 5 mM Hepes⋅NaOH (pH 7.4). List L/M-EPC7 patch-clamp amplifier (List Medical, Darmstadt, Germany) was used to record channel current. Channel currents were low-pass-filtered at 1 kHz by an eight-pole Bessel filter (902LPF, Frequency Devices, Haverhill, MA), and recordings were digitized at a sampling rate of 44 kHz by the use of a modified digital data recorder (Instrutech, Great Neck, NY) and were stored on videotape (JVC HR-J400U). For analysis, data were acquired and stored on hard disk (Gateway 2000 4DX) at a sampling rate of 5 kHz and analyzed with pclamp software system (version 6.0, Axon Instruments, Foster City, CA). Before patch-clamp study, oocytes were treated with hypertonic solution containing 220 mM methylglucamine, 220 mM aspartic acid, 2 mM MgCl2, 10 mM EGTA, and 10 mM Hepes⋅NaOH (pH 7.2), to remove the vitellin membrane.
PKA Assay.
Eighty oocytes were homogenized with a Teflon Erlenmeyer homogenizer in 1 ml of lysis buffer [20 mM Tris⋅HCl, pH 7.5/0.5 mM EDTA/0.5 mM EGTA/leupeptin (25 μg/ml)/aprotinin (25 μg/ml)/10 mM 2-mercaptoethanol/300 mM sucrose]. The PKA activity of the oocyte homogenate was determined by the P81 phosphocellulose cation-exchange paper assay (16). In brief, activity measurements were carried out in reaction buffer [40 mM Hepes⋅NaOH, pH 6.8/10 mM MgCl2/100 μM [32P]ATP (500 cpm/pmol)/DTT (6 mg/ml)] in the presence and absence of cAMP by using kemptide as substrate.
Expression of RII Subunit of PKA.
A plasmid containing the RII of PKA, a gift from John Scott (Vollum Institute, Portland, OR), was transfected into Escherichia coli JM 109. RII was purified and phosphorylated, as described by Scott (17), with catalytic subunit of PKA (5 mU; Boehringer Mannheim) in 20 μl of 50 mM Tris⋅HCl (pH 7.5), 10 mM magnesium acetate, 150 mM NaCl, 50 μCi of [32P]ATP (Amersham; 6,000 Ci/mmol). After a 1-hr incubation at 30°C, samples were desalted on Pierce GF-5 (5 ml) columns. 32P labeled RII was stored at 4°C.
32P-Labeled RII Overlay Assay.
Membrane fractions from kidney and oocytes (20 μg) were separated by SDS/PAGE and electrophoretically transferred to nitrocellulose membranes. Nitrocellulose membranes were used for the 32P - RII overlay assay, as described by Scott (17).
Two-Electrode Whole-Cell Voltage-Clamp Technique.
A Warner oocyte clamp OC-725C was used to measure the whole-cell K+ current. Voltage and current microelectrodes were filled with 1 M KCl and had a resistance of less than 2 MΩ, and the series resistance was compensated. The current was recorded on a chart recorder (Gould TA240). After the current reached a steady state for 1 min, 2 mM Ba+2 was used to determine the Ba+2-sensitive K+ current. Oocytes were superfused with the bath solution containing 150 mM KCl, 5 mM MgCl2, 1 mM EGTA, and 5 mM Hepes⋅NaOH (pH 7.4) and were clamped at −60 mV. Forskolin and 8-bromo-cAMP (8-Br-cAMP) were purchased from Sigma and added directly to the bath to the final concentration. Before the experiments were completed, 2 mM Ba2+ was applied to determine the Ba2+-sensitive K+ current.
Data are presented as the mean ± SEM. Student’s t test was used to determine the significance.
RESULTS
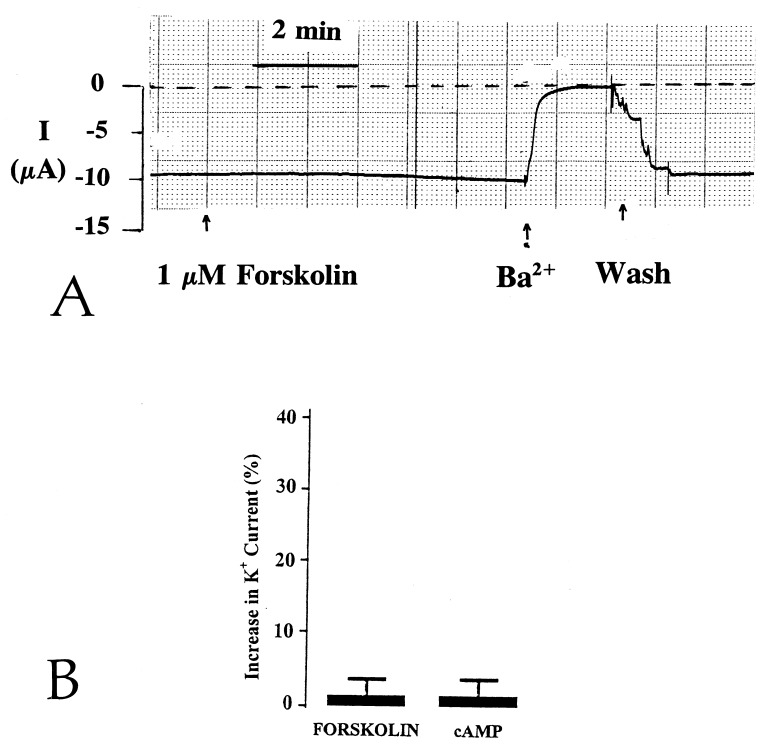
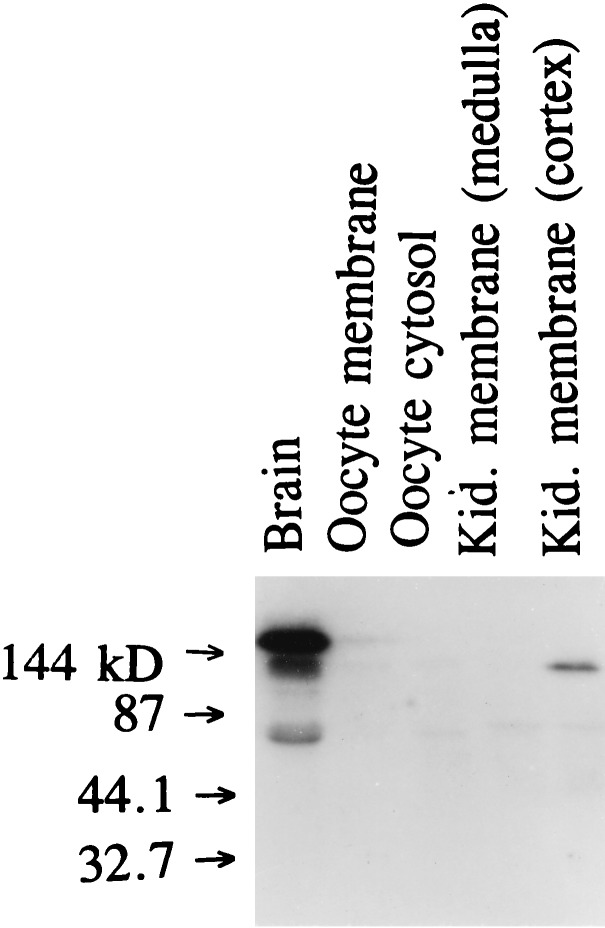
In inside-out patches, ROMK1 channels have been shown to be stimulated by PKA-induced phosphorylation (7). We extended the study to investigate whether stimulation of PKA can also increase the activity of ROMK1 channels in intact cells. Fig. 1A is a representative tracing showing the effect of 1 μM forskolin on Ba+2-sensitive whole-cell K+ current by using the two-electrode voltage clamp technique. Forskolin failed to activate ROMK1 channels expressed in oocytes. To assess whether the absence of a response of ROMK1 channels to forskolin is the result of failure to increase intracellular cAMP levels, we examined the effect of 100 μM 8-Br-cAMP on ROMK1 channels. Like forskolin, 8-Br-cAMP had no significant effect on whole-cell K+ current (Fig. 1B). Under these conditions, PKA activity in oocytes increased 10-fold (data not shown). It has recently been suggested that AKAPs mediate the effect of PKA on ion channels (12–14). Therefore, we explored the possibility that the absence of AKAP is responsible for the failure of ROMK1 channels to be stimulated by PKA. We initially used a RII overlay assay to investigate whether AKAP is expressed in the membrane of oocytes. Fig. 2 is a representative RII overlay assay showing that there is no detectable RII-binding protein in the membrane of oocytes. In contrast, we could identify a RII-binding protein with a molecular mass of 100–120 kDa in the membrane obtained from the kidney cortex. This result is consistent with observations that stimulation of PKA increases the activity of the native ROMK-like channels (1).
Figure 1.
(A) Representative trace showing the effects of 1 μM forskolin on the Ba2+-sensitive channel current measured by the two-electrode voltage-clamp technique. The oocytes were clamped at −60 mV. The dotted line indicates the closed current. Addition of forskolin and Ba2+ is indicated by arrows. (B) Effect of 1 μM forskolin and 100 μM 8-Br-cAMP (cAMP) on the Ba2+-sensitive K+ current.
Figure 2.
Autoradiograph showing the results of 32P-labeled RII overlay assay. The RII-binding protein with a molecular mass of 100-120 kDa in brain and kidney membranes (cortex) is shown.
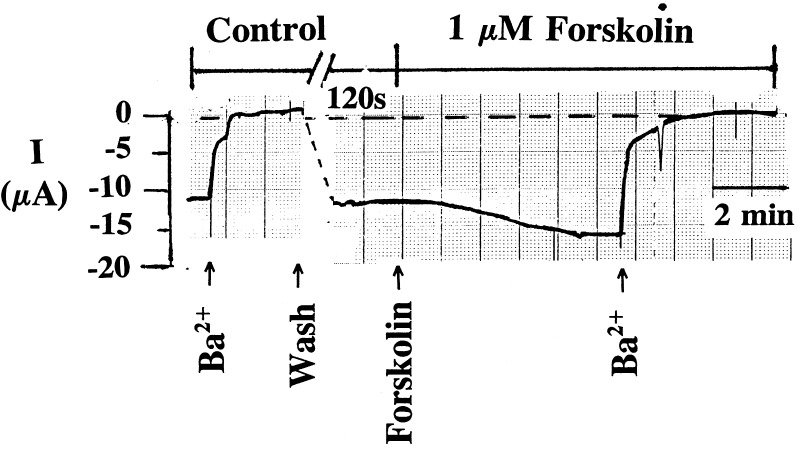
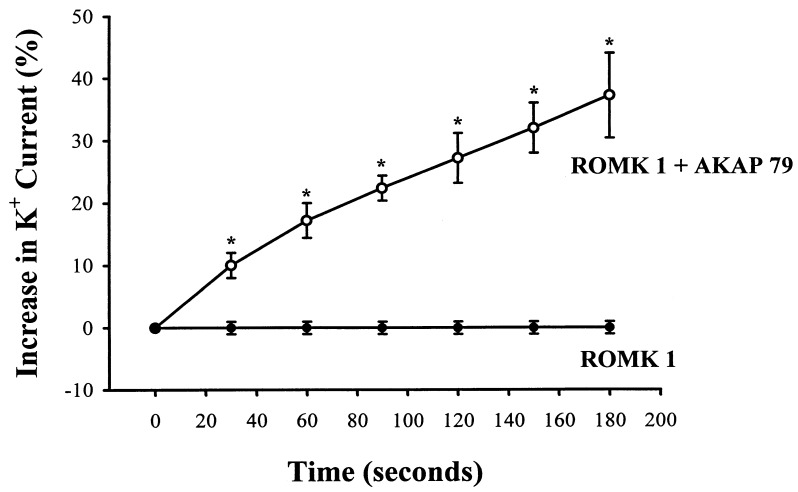
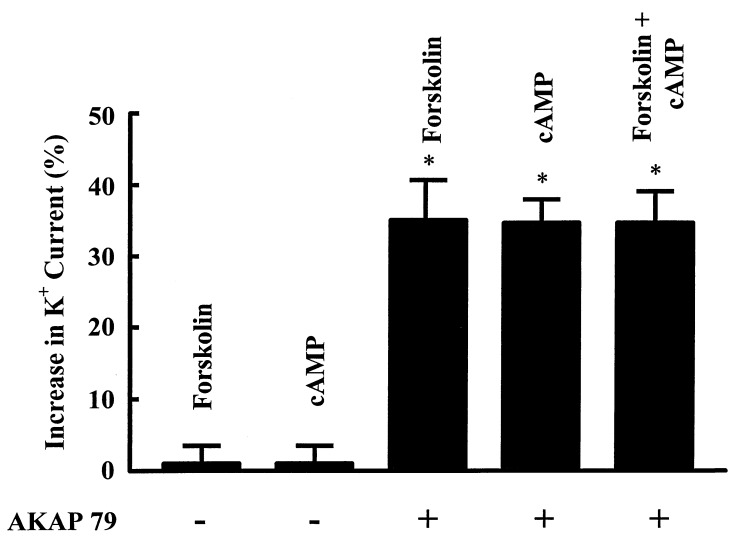
The possibility that an AKAP is required for the stimulatory effect of PKA on ROMK1 channels was explored by coexpressing the ROMK1 channels with AKAP79. Although AKAP79 is not expressed in the kidney, we reasoned that AKAP79, a membrane-associated anchoring protein, would restore the sensitivity of ROMK1 channels to forskolin. The expression of AKAP79 was confirmed by Western blot analysis (data not shown). In contrast to results observed in the control oocytes (Fig. 1), forskolin significantly increased Ba+2-sensitive K+ current in oocytes injected with a cRNA encoding AKAP79 and ROMK1 channels (Fig. 3). Fig. 4 summarizes the results of 20 experiments obtained from the control oocytes and from oocytes coexpressing the ROMK1 channels with AKAP79. It is apparent that forskolin significantly stimulates the ROMK1 channels by 35 ± 5% within 3 min in oocytes coexpressing AKAP79 and ROMK1 but not oocytes expressing ROMK1 channels alone.
Figure 3.
Representative trace showing the effect of 1 μM forskolin on the Ba2+-sensitive K+ current when oocytes were injected with cRNAs encoding the AKAP79 and ROMK1 channel. The dotted line indicates the closed level of channel current and arrows indicate where the agents were added to the bath.
Figure 4.
Effect of 1 μM forskolin on ROMK1 channel current with coexpression of AKAP79 (○) and without AKAP79 (•). Asterisks indicate that data were significantly different from the control value.
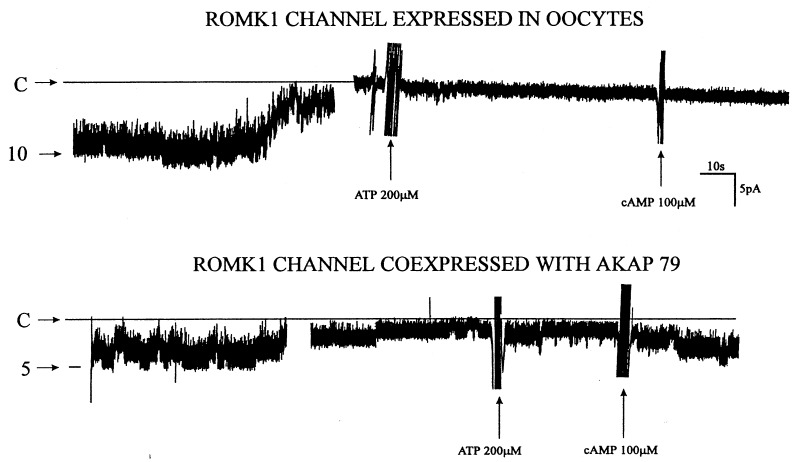
To examine whether the effect of forskolin is the result of increasing intracellular cAMP levels, we examined the effect of 8-Br-cAMP on channel activity. Fig. 5 summarizes the results obtained from 10 experiments: addition of 100 μM 8-Br-cAMP increased channel current by 33.5 ± 5%. Moreover, in the presence of 8-Br-cAMP, application of forskolin did not further increase the ROMK1 channel current. These findings suggest that the effects of cAMP and forskolin are not additive and that forskolin increases channel activity via a cAMP-dependent pathway. To further investigate the stimulatory effect of cAMP on ROMK1 channels, we used the patch-clamp technique to study the effect of cAMP on channel activity in inside-out patches in oocytes with or without coexpression of AKAP79. We confirmed the previous finding that the channel activity decreased significantly (channel run down) upon excision of patch membranes in a Mg+2-containing bath solution. In the absence of AKAP79, addition of 100 μM cAMP and 100 μM Mg+2ATP failed to restore ROMK1 channel activity in all 21 patches in which channel activity was observed in cell-attached patches (Fig. 6 and Table 1). In contrast, when the ROMK1 channel and AKAP79 were coexpressed, addition of 100 μM cAMP and 100 μM Mg+2ATP restored channel activity after run down in six experiments out of 10 patches (Fig. 6 and Table 1).
Figure 5.
Effects of 1 μM forskolin and 100 μM 8-Br-cAMP (cAMP) on ROMK1 channel current when oocytes were injected with cRNAs for AKAP79 and ROMK1 channels. Asterisks indicate that data are significantly different from the control value.
Figure 6.
Channel recording showing the effects of 100 μM MgATP and 100 μM cAMP on ROMK1 channel activity when oocytes were injected with ROMK1 channel alone (Upper) and injected with both ROMK1 channel and AKAP79 (Lower). The closed level of channel current (C) is indicated, and the number of channels in the patch is also indicated. Experiments were performed in inside-out patches and the cell membrane potential was −30 mV. The gap in the trace is 60 s.
Table 1.
Effects of ATP and cAMP on ROMK1 channels
| No. with positive response to ATP + cAMP | Total no. of patches | |
|---|---|---|
| ROMK | 0 | 21 |
| ROMK + AKAP79 | 6 | 10 |
MgATP (100 μM) and cAMP (100 μM) were used. Total no. of patches indicates numbers of patches in which channel activity was present in cell-attached patches but run-down after forming inside-out patches. Positive response means that addition of cAMP + ATP can restore the channel activity.
DISCUSSION
The ROMK channel is the key component of the native low-conductance ATP-sensitive K+ channel in the CCD and TAL (1). The CCD is responsible for K+ secretion, and the K+ secretion is achieved by active uptake of K+ by the basolateral Na,K-ATPase and then by K+ diffusing across the apical membrane via the ROMK-like low-conductance K+ channel. Therefore, the ROMK channels should play a key role in K+ secretion (18). In addition, ROMK channels play a key role in K+ recycling in the TAL (1). Genetic studies have demonstrated that the defective gene encoding ROMK channels results in an abnormality of salt transport in Henle’s loop (Bartter’s syndrome) (19, 20).
Vasopressin has been shown to be involved in the regulation of the native low-conductance K+ channel in the TAL and CCD (21, 22). We have shown (21, 22) that addition of vasopressin increased the activity of the native low-conductance K+ channel in the TAL and CCD. The effect of vasopressin is mediated by a cAMP-dependent pathway because application of cAMP analogs mimics the effect of vasopressin (21). That vasopressin stimulates the apical K+ conductance in the TAL has been reported in several studies using microelectrodes and vesicles (23, 24).
ROMK channels have three putative serine PKA phosphorylation sites (2), and Xu et al. (25) have shown that these sites can be phosphorylated in vitro. Moreover, addition of exogenous PKA stimulated the ROMK1 channels, (7) and deletion of one serine PKA phosphorylation site diminished the K+ current (25). However, the effect of PKA on ROMK1 channels can be observed only in excised patches (7), but not in intact cells, when ROMK1 is expressed in oocytes. In contrast, we observed (21, 22) the effect of PKA on native K+ channels in both cell-attached and inside-out patches. Three lines of evidence indicate that absence of AKAP is responsible for the inability of cAMP or forskolin to stimulate ROMK1 channels. (i) RII overlay assay indicates the presence of AKAPs in membranes isolated from kidney cortex but not in membranes of oocytes. (ii) The effect of forskolin and cAMP on ROMK1 channels can be reestablished by coexpression of AKAP79 and ROMK1 channels. (iii) Finally, in inside-out patches, cAMP can restore the channel activity in membrane obtained from oocytes coexpressing AKAP79 and ROMK1 but not from those expressing ROMK1 alone. Although we can restore the channel activity in only 60% of patches with run-down channels in oocytes coexpressing AKAP79 and ROMK channels, it is possible that AKAP79 is not located close to the ROMK channel in those patches. This observation is consistent with findings that in less than 50% of patches from the native tissue could addition of cAMP and MgATP reestablish the activity of the ROMK-like K+ channel in inside-out patches after channel run-down (26).
Anchoring of PKA with AKAP is an efficient way to control the spatiotemporal resolution of PKA-induced phosphorylation (10). It has been suggested that the function of AKAP may be to increase local concentration of the kinase and to facilitate phosphorylation of the target proteins (10). In addition to being involved in the regulation of ion channels, AKAP has been shown to play an important role in insulin secretion (27). Several types of AKAP have been characterized and cloned (28–30). AKAP79 is a member of this growing family and has been shown to bind not only PKA but also protein kinase C and protein serine/threonine phosphatase 2B (8). Although AKAP79 is primarily expressed in the neuronal tissue but not in the kidney, coexpression of AKAP79 restores the response of ROMK1 channels to stimulation of PKA. This may be related to the fact that ROMK channels have been shown to be expressed in the brain (31). Thus, it is possible that AKAP79 may be involved in mediating PKA-induced phosphorylation of ROMK channels in the brain. The present study revealed that there is at lease one renal version of AKAP. Further study is needed to explore the nature of the renal AKAP and to examine the effect of stimulating PKA in cells coexpressing renal AKAP and ROMK channels.
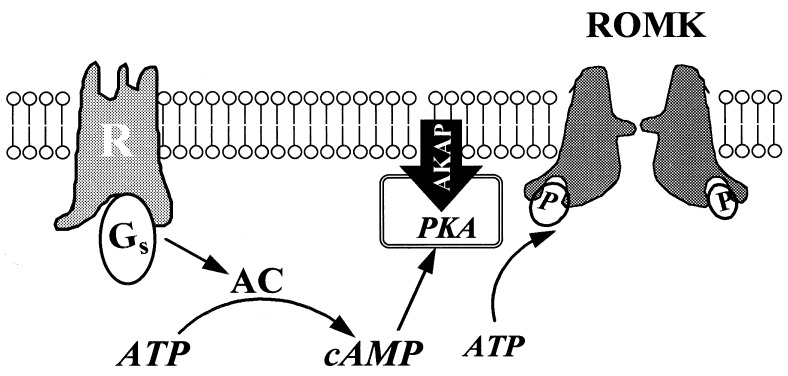
Fig. 7 is a cell model illustrating the role of AKAP in mediating the stimulatory effect of PKA on ROMK-like K+ channels. Stimulation of the Gs-coupled receptor increases intracellular cAMP levels and, accordingly, activates PKA, which is associated with the membrane through AKAP in close proximity to the ROMK-like K+ channel. Consequently, the ROMK channel is phosphorylated by PKA. Therefore, the specificity of the PKA effect is partially determined by the presence of AKAPs.
Figure 7.
Schema illustrating the role of AKAP in mediating the PKA stimulation of ROMK1-like channels in the kidney. Stimulation of the receptor’s (R) coupling to the G-protein (Gs) activates adenylate cyclase (AC), which increases cAMP production. cAMP can consequently stimulate PKA, which binds to the cell membrane through the AKAP. Accordingly, the ROMK channel is phosphorylated (P).
Acknowledgments
We thank Dr. J. D. Scott for providing the cDNA encoding AKAP79 and RII. We thank M. Steinberg for help in preparation of the manuscript. The work is supported by National Institutes of Health Grant DK-37605 (to S.C.H.), HL-4489301 (to K.M.L.), DK47402 (W.H.W.), and P01HL34300 (W.H.W.). K.M.L. is the recipient of an Establish Investigatorship of American Heart Association.
ABBREVIATIONS
- AKAP
A kinase anchoring protein
- PKA
protein kinase A
- ROMK1
renal outer medulla K channel
- TAL
thick ascending limb
- CCD
cortical collecting duct
References
- 1.Wang W H, Hebert S C, Giebisch G. Annu Rev Physiol. 1997;59:413–436. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
- 2.Ho K, Nichols C G, Lederer W J, Lytton J, Vassilev P M, Kanazirska M V, Hebert S C. Nature (London) 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 3.Lee W S, Hebert S C. Am J Physiol. 1995;268:F1124–F1131. doi: 10.1152/ajprenal.1995.268.6.F1124. [DOI] [PubMed] [Google Scholar]
- 4.Xu J Z, Hall A E, Peterson L N, Bienkowski M J, Eessalu T E, Hebert S C. Am J Physiol. 1997;273:F739–F748. doi: 10.1152/ajprenal.1997.273.5.F739. [DOI] [PubMed] [Google Scholar]
- 5.Chepilko S, Zhou H, Sackin H, Palmer L G. Am J Physiol. 1995;268:C389–C401. doi: 10.1152/ajpcell.1995.268.2.C389. [DOI] [PubMed] [Google Scholar]
- 6.Palmer L G, Choe H, Frindt G. Am J Physiol. 1997;273:F404–F410. doi: 10.1152/ajprenal.1997.273.3.F404. [DOI] [PubMed] [Google Scholar]
- 7.McNicholas C M, Wang W, Ho K, Hebert S C, Giebisch G. Proc Natl Acad Sci USA. 1994;91:8077–8081. doi: 10.1073/pnas.91.17.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klauck T M, Faux M C, Labudda K, Langeberg L K, Jaken S, Scott J D. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 9.Rubin C S. Biochim Biophys Acta. 1994;1224:467–479. [PubMed] [Google Scholar]
- 10.Mochly-Rosen D. Science. 1995;268:247–250. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- 11.Corbin J D, Sugden P H, Lincoln T M, Keely S L. J Biol Chem. 1977;252:3854–3861. [PubMed] [Google Scholar]
- 12.Gao T Y, Yatani A, Dell’Acqua M L, Sako H, Green S A, Dascal N, Scott J D, Hosey M M. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 13.Johnson B D, Scheuer T, Catterall W A. Proc Natl Acad Sci USA. 1994;91:11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenmund C, Carr D W, Bergeson S E, Nilaver G, Scott J D, Wesrbrook G L. Nature (London) 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 15.Macica C M, Yang Y H, Hebert S C, Wang W H. Am J Physiol. 1996;271:F588–F594. doi: 10.1152/ajprenal.1996.271.3.F588. [DOI] [PubMed] [Google Scholar]
- 16.Hoyt C H, Oh C J, Beekman J B, Litchfield D W, Lerea K M. Blood. 1994;12:3517–3523. [PubMed] [Google Scholar]
- 17.Scott J D, Stofko R E, McDonald J R, Comer J D, Vitalis E A, Mangili J A. J Biol Chem. 1990;265:21561–21566. [PubMed] [Google Scholar]
- 18.Giebisch G. Kidney Int. 1995;48:1004–1009. doi: 10.1038/ki.1995.382. [DOI] [PubMed] [Google Scholar]
- 19.Simon D B, Karet F E, Rodriguez J, Hamdan J H, DiPietro A, Trachtman H, Sanjad S A, Lifton R P. Nat Genet. 1996;14:152–156. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 20.Simon D B, Nelson-Williams C, Bia M J, Ellison D, Karet F E, Molina A M, Vaara I, Iwata F, Cushner H M, Koolen M, Gainza F, Gitelman H J, Lifton R P. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 21.Wang W H. Am J Physiol. 1994;267:F599–F605. doi: 10.1152/ajprenal.1994.267.4.F599. [DOI] [PubMed] [Google Scholar]
- 22.Cassola A C, Giebisch G, Wang W. Am J Physiol. 1993;264:F502–F509. doi: 10.1152/ajprenal.1993.264.3.F502. [DOI] [PubMed] [Google Scholar]
- 23.Hebert S C, Friedman P A, Andreoli T E. J Membr Biol. 1984;80:201–219. doi: 10.1007/BF01868439. [DOI] [PubMed] [Google Scholar]
- 24.Reeves W B, McDonald G A, Mehta P, Andreoli T E. J Membr Biol. 1989;109:65–72. doi: 10.1007/BF01870791. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z C, Yang Y, Hebert S C. J Biol Chem. 1996;271:9313–9319. doi: 10.1074/jbc.271.16.9313. [DOI] [PubMed] [Google Scholar]
- 26.Wang W H, Giebisch G. J Gen Physiol. 1991;98:35–61. doi: 10.1085/jgp.98.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lester L B, Langeberg L K, Scott J D. Proc Natl Acad Sci USA. 1997;94:14942–14947. doi: 10.1073/pnas.94.26.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr D W, Stofko-Hahn R E, Fraser I D C, Cone R D, Scott J D. J Biol Chem. 1992;267:16816–16823. [PubMed] [Google Scholar]
- 29.Sarkar D, Erlichmann J, Rubin C S. J Biol Chem. 1984;259:9840–9846. [PubMed] [Google Scholar]
- 30.Murphy B J, Scott J D. Trends Cardiovasc Med. 1998;8(2):89–95. doi: 10.1016/S1050-1738(97)00131-X. [DOI] [PubMed] [Google Scholar]
- 31.Kondo C, Isomoto S, Matsumoto S, Yamada M, Horio Y, Yamashita S, Takemura-Kameda K, Matsuzawa Y, Kurachi Y. FEBS Lett. 1996;399:122–126. doi: 10.1016/s0014-5793(96)01302-6. [DOI] [PubMed] [Google Scholar]