Abstract
Recent genetic studies have implicated pro-inflammatory chemokines and chemokine receptors in atherogenesis. Studies at the molecular and cellular level have suggested specific atherogenic mechanisms for two chemokine-chemokine receptor pairs, CCL2:CCR2 and CX3CL1:CX3CR1, involving differential receptor regulation by the transcription factor PPARγ. This pathway is triggered by oxidized pro-atherogenic lipids, such as oxidized LDL and linoleic acid derivatives, which promote differentiation of CCR2hiCX3CR1lo human monocytes to CCR2loCX3CR1hi macrophages that adhere to coronary artery smooth muscle cells in a CX3CR1- and PPARγ-dependent manner. Switching CX3CR1 on and CCR2 off in vivo may result in cessation of CCR2-dependent migration and activation of CX3CR1-dependent retention that together may promote foam cell accumulation in the vessel wall.
Introduction
Atherogenesis is now widely accepted to involve not only progressive buildup of oxidized lipids in the arterial wall, but also chronic inflammation in which progression towards a rupture-prone, unstable atherosclerotic plaque is driven in part by leukocytes infiltrating the vascular subendothelium (Ross 1999). The molecular basis for leukocyte accumulation in the vessel wall has not been clearly delineated; however, there is increasing evidence implicating specific adhesion molecules and members of the chemokine superfamily of leukocyte chemoattractants (Charo and Taubman 2004, Weber et al. 2004, Ludwig and Weber 2007). Chemokines are divided into four major subfamilies--C, CC, CXC, CX3C--based on the number and positioning of conserved cysteines in the amino-terminal portion of the protein chain, and act at seven-transmembrane domain G protein-coupled receptors (GPCRs). There are ~50 human chemokines and at least 18 human chemokine receptors (Murphy 2000). Recent work has shown that oxidized LDL (oxLDL) and oxidized lipid components of LDL found in the vessel wall may directly or indirectly influence expression of certain chemokines and chemokine receptors (Han et al. 2000, Lei et al. 2002, Barlic et al. 2006). In this review we focus on a novel pathway by which two pro-atherogenic chemokine receptors, CCR2 and CX3CR1, are differentially regulated by oxidized lipids, and discuss the potential functional significance of this in atherosclerosis.
CCR2-CCL2 and CX3CR1-CX3CL1 in Atherosclerosis
Monocytes and mature macrophages are the main leukocyte subsets that accumulate at lipid-laden vascular sites in atherosclerosis (Ross 1999). Monocyte chemoattractant protein-1 (MCP-1, or CCL2 in the systematic chemokine nomenclature) has been detected in macrophage-rich areas bordering the lipid core, as well as on endothelial and smooth muscle cells in human and mice atherosclerotic lesions (Yla-Herttuala et al. 1991, Nelken et al. 1991, Rayner et al. 2000). In mouse, direct evidence supporting a role for CCL2 in atherogenesis has been obtained through targeted gene disruption in atherosclerosis-prone mouse strains (low-density lipoprotein receptor-/- [ldlr−/−] or transgenic apolipoprotein B). On these backgrounds, lesion size and plaque macrophage content were decreased by >60% in mice lacking CCL2 (Gu et al. 1998, Gosling et al. 1999). Conversely, over-expression of CCL2 accelerated atherosclerosis in irradiated hypercholesterolemic apolipoprotein E (apoE−/−) recipients (Aiello et al. 1999). A single nucleotide polymorphism (SNP) -2518G (alternatively -2578G) in the regulatory region of human CCL2, which causes increased promoter activity and elevated circulating CCL2 levels, has provided an opportunity to test the role of CCL2 in human atherosclerosis. Consistent with the mouse results, this SNP has been associated with a modest increased risk of myocardial infarction in two independent studies, including the Framingham Heart Study Offspring Cohort (Szalai et al. 2001, McDermott et al. 2005).
CCL2 activates T cell and monocyte chemokine receptor CCR2, which has been identified directly on foam cells in human atherosclerotic lesions (Charo and Taubman 2004, Weber et al. 2004, Ludwig and Weber 2007). Genetic evidence in both mouse and man support a functional role for CCR2 in atherogenesis. In mice, both genetic inactivation of ccr2 on an apoE−/− atherogenic background (Boring et al. 1998) and transplantation of bone marrow from ccr2−/− mice into irradiated apoE3-Leiden recipients (Guo et al. 2003) results in decreased susceptibility to atherosclerosis. In humans, the SNP CCR2-V64I has been associated with increased risk of myocardial infarction and left ventricular heart failure (Ortlepp et al. 2003). A caveat in interpreting this result is that CCR2-V64I has not been shown to directly affect receptor function; instead, it may be part of a haplotype bearing another unidentified functionally important genetic change.
CX3CR1 and its ligand CX3CL1 (also known as fractalkine) have also been implicated in atherosclerosis by genetic evidence. CX3CL1 is an atypical multimodular chemokine that exists both in membrane-tethered and soluble forms. The immobilized form consists of a chemokine domain anchored to the plasma membrane through an extended mucin-like stalk, followed by a transmembrane helix and an intracellular domain (Bazan et al. 1997). Full-length CX3CL1 functions as an intercellular adhesion molecule that mediates integrin-independent cell capture by binding to CX3CR1 on target cells (Fong et al. 1998). A disintegrin-like metalloproteinase has been identified that cleaves the chemokine domain of CX3CL1 (Garton et al. 2001), which may promote trafficking of CX3CR1+ monocytes, platelets, NK cells, NK-T cells, T cells and dendritic cells to sites of inflammation (Imai et al. 1997).
Neither CX3CL1 nor CX3CR1 has been found in normal mouse or human arterial wall. However, in the context of atherosclerosis, both molecules are expressed on both foam cells and coronary artery smooth muscle cells, but not endothelial cells, in both species (Wong et al. 2002, Lesnik et al. 2003). Targeted disruption of cx3cl1 or cx3cr1 does not affect viability or fertility or lead to spontaneous infections in mice. However, deletion of cx3cr1 in apoE−/− mice (Lesnik et al. 2003, Combadiere et al. 2003) or cx3cl1 in either ldlr−/− or apoE−/− mice (Teupser et al. 2004) has been reported to decrease susceptibility to atherosclerosis by ~50%. Two independently derived lines of cx3cr1−/−apoE−/− mice both showed decreased lesion formation with fewer macrophages infiltrating plaques in the aortic root (Lesnik et al. 2003, Combadiere et al. 2003), whereas the cx3cl1−/−apoE−/− strain had dramatically decreased atherosclerosis in the brachiocephalic artery, and cx3cl1−/−ldlr−/− mice displayed smaller lesions in both the aortic root and the brachiocephalic artery (Teupser et al. 2004).
Translating this to man has been facilitated by discovery of two common human CX3CR1 non-synonymous coding region SNPs, named I249V and T280M for the amino acid changes they cause, which strongly affect function. These SNPs are in strong linkage disequilibrium, and for simplicity we refer to the most common variant receptor, which contains both V249 and M280, as CX3CR1-M280. In retrospective cohort studies these polymorphisms have been consistently associated with reduced prevalence of disease endpoints (Odds Ratio = 0.6-0.7), including coronary endothelial dysfunction and physical coronary artery stenosis in a National Heart, Lung and Blood Institute cardiac catheterization cohort (McDermott et al. 2001), reduced prevalence of acute coronary events in the Accidents Coronaires Aigus Bichat cohort (Moatti et al. 2001), reduced progression of carotid atherosclerosis (Ghilardi et al. 2004) and association with lower risk of cardiovascular events in the Framingham Heart Study Offspring cohort (McDermott et al. 2003). Consistent with the loss-of-function results for mouse CX3CR1, McDermott et al. found that transfected cells expressing recombinant CX3CR1-M280 were defective both in binding the iodinated chemokine domain of CX3CL1 and in adhering to immobilized endothelial cell-expressed recombinant CX3CL1 under conditions of physiologic shear (McDermott et al. 2003). An independent study by Daoudi et al. found that CX3CR1-M280, tested in both transfected cells and at natural abundance in primary cells from homozygotes, promoted substantially greater intercellular CX3CL1-dependent adhesion than wild-type CX3CR1 (Daoudi et al. 2004). Further work will be needed to clarify this discrepancy, which is very important from a translational perspective; however, regardless of whether CX3CR1-M280 functions as a gain- or loss-of-function mutant, it has been consistently reported to function abnormally and to be associated with reduced risk of cardiovascular disease in man.
In both humans and mice, monocytes are a heterogeneous population of circulating leukocytes that may be separated into two major subsets with respect to chemokine receptor expression pattern: CCR2+CX3CR1low (classical monocytes) and CCR2−CX3CR1high (non-classical monocytes) (Geissmann et al. 2003). Recent studies from apoE−/− mice suggest that phagocyte heterogeneity in plaques is linked to distinct types of entering monocytes. While CCR2−CX3CR1high monocytes, which are less prevalent, utilize CCR5 to enter plaques, CCR2+CX3CR1low monocytes, the predominant monocyte subset, require CCR2, CCR5 and CX3CR1 to accumulate within plaques (Tacke et al. 2007). These results suggest that CCR2 and CX3CR1 both support the recruitment of classical monocytes into the arterial wall.
Regulation of CCR2 and CX3CR1 Expression and Function in the Atheromatous Microenvironment – the Role of PPARγ
Monocyte recruitment to lesion-prone areas involves not only pathological changes in the arterial wall but also functional adjustments of the entering monocytes. The atheromatous microenvironment includes a specific mix of oxLDL and its free lipid constituents, which have been shown to stimulate monocyte recruitment and inhibit macrophage motility (Gerrity 1981, Gerrity et al. 1985). These properties of oxLDL and its derivatives may help explain how monocytes are recruited to and trapped within plaques.
It has been clearly demonstrated that oxLDL regulates monocyte gene expression (Tontonoz et al. 1998); thus, the mechanism utilized by oxLDL to induce functional changes of monocytes may include oxidized lipid-dependent regulation of monocyte chemoattractant receptors. In this regard, stimulation of human monocytes with oxLDL or end-point oxidation derivatives of linoleic acid found in high amounts in human atherosclerotic lesions (9-hydroxy-10E,12Z-octadecadienoic acid ester [9-HODE] and 13-hydroxy-9Z,11E-octadecadienoic acid ester [13-HODE]) (Glavind and Hartmann 1951, Harland et al. 1973, Jira et al. 1998) specifically induced differentiation of CCR2highCX3CR1low monocytes to CCR2lowCX3CR1high macrophages that strongly adhered to CX3CL1+ primary human coronary artery smooth muscle cells (CASMCs) under static conditions (Barlic et al. 2006) (Figure 1). Adhesion of macrophages to CASMCs was mediated directly and predominantly by CX3CR1. Furthermore, effects of oxLDL and its end-point oxidation products on CX3CR1 and CCR2 expression was mediated by peroxisome-proliferator activated receptor γ (PPARγ), since knockdown of PPARγ with specific sRNAi reversed the oxLDL-induced chemokine receptor switch and dramatically reduced CX3CR1-mediated macrophage adhesion to CASMCs (Barlic et al. 2006) (Figure 1). This observation suggests that in atherogenesis oxidized lipid-driven activation of macrophage PPARγ in the intima results in a proadhesive chemokine receptor switch-CCR2 off, CX3CR1 on-causing cessation of CCR2-dependent migration and activation of CX3CR1-dependent retention mechanisms, which together promote macrophage accumulation in vessel wall. These results suggest a second function in atherosclerosis for CX3CR1, which has also been shown to promote monocyte recruitment into plaques (Tacke et al. 2007). Since CX3CR1 and its ligand CX3CL1 are both expressed by foam cells and smooth muscle cells in atherosclerotic plaques (Wong et al. 2002, Lesnik et al. 2003), PPARγ activity may also support CX3CR1-dependent homotypic adhesive interactions of these cell types in plaque, a possibility that has not yet been addressed experimentally.
Figure 1.
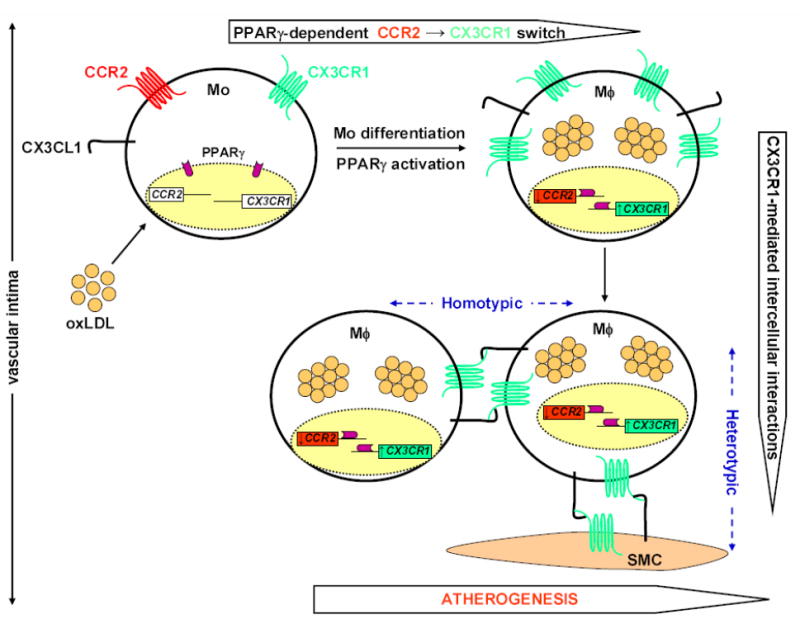
A pro-adhesive PPARγ-mediated chemokine receptor switch, CCR2→CX3CR1, in atherosclerosis. LDL diffuses from the blood into the vascular intima, where it undergoes oxidative modifications forming randomly distributed extracellular pools of oxLDL and its stable linoleic acid metabolites. Circulating blood monocytes expressing both CCR2 and CX3CR1 invade inflamed arteries in response to pro-inflammatory cytokines and chemoattractants produced by vascular endothelium through a multi-step process that includes selectin-mediated rolling, integrin-firm arrest, spreading and diapedesis. After entering the subendothelial space, the atheromatous microenvironment rich in oxLDL stimulates monocyte differentiation into foamy macrophages that are the predominant cell population in early atherosclerotic lesions. Furthermore, ingestion of oxLDL by monocytes activates the transcription factor PPARγ, which not only participates in maturation of monocytes to macrophages, but also promotes simultaneous downregulation of CCR2 and upregulation of the adhesion chemokine receptor CX3CR1 expression, thus promoting cessation of macrophage migration, and their capture and retention in the plaque. Furthermore, since CX3CR1 and its ligand CX3CL1 are expressed by various cell types in lesions, this pro-adhesive CCR2→CX3CR1 switch may support formation of numerous heterotypic and homotypic intercellular interactions maintained by a pro-atherogenic CX3CL1-CX3CR1 axis that may contribute towards organization of cells in plaque. Mo, monocyte; Mϕ, foamy macrophage; oxLDL, oxidized LDL; SMC, smooth muscle cell.
PPARγ is widely expressed, including in the lipid core of human atherosclerotic lesions, where it has been co-localized with markers specific for endothelial cells, foam cells and smooth muscle cells (Ricote et al. 1998a). A variety of substances function as ligands for PPARγ, including fatty acids and eicosanoids, components of oxLDL and oxidized alkyl phospholipids including lysophosphatidic and nitrolinoleic acid, and 15-deoxy-Δ12,14-prostaglandin J2 (Knouff and Auwerx 2004). In addition, thiazolidinediones (TZDs) are synthetic PPARγ agonists able to mimic the effects of oxLDL and oxidized linoleic acid metabolites on CCR2 and CX3CR1 expression in monocytes (Barlic et al. 2006).
PPARγ as a Target in Atherosclerosis
The genetic results reviewed above suggest that blocking the CCL2:CCR2 and CX3CL1:CX3CR1 axes alone or in combination could be beneficial in the setting of atherosclerotic cardiovascular disease. So far no such blocking agents have been entered into clinical trials for this indication, and there are major obstacles that would confront any such effort, such as the difficulty of monitoring efficacy at the site of action, the vessel wall. PPARγ, on the other hand, has a long track record as a safe therapeutic target of TZDs (Li and Palinski 2006). In particular, recently published interim results of the RECORD trial (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes), a long-term, multicenter, randomized and open-label study involving 4447 patients with type 2 diabetes mellitus (T2DM), did not find an association of this TZD with increased risk of myocardial infarction or death from cardiovascular causes (Home et al. 2007), in contrast to results from smaller studies (Nissen and Wolski 2007). On the contrary, surrogate marker studies mainly of diabetic patients have supported possible vascular benefits of TZDs, including decreased carotid artery intimal and medial thickening (Sidhu et al. 2003), improved endothelial reactivity (Campia et al. 2006) and lower levels of inflammatory markers and mediators (Meisner et al. 2006), even in nondiabetics (Rios-Vazquez et al. 2006). Consistent with this, treatment of atherosclerosis-prone ldlr−/− mice with synthetic PPARγ agonists results in relative resistance to atherosclerosis (Verreth et al. 2006).
Given the pleotropic effects of PPARγ it is difficult to pinpoint the mechanism(s) of action driving the benefits of TZDs in atherosclerosis. With regard to chemokine receptors, their ability to downregulate monocyte-macrophage CCR2 is likely to be beneficial whereas this might be offset by CX3CR1 upregulation. Also complicating the issue, PPARγ is also important in lipid homeostasis (lipoprotein uptake and cholesterol efflux) and energy balance, and governs physiological processes such as adipocyte differentiation, glucose homeostasis, inflammatory responses and maturation of macrophages into foam cells (Lehrke and Lazar 2005). Furthermore, disruption of PPARγ in myeloid cells impairs alternative macrophage activation and pre-disposes these animals to development of diet-induced obesity, insulin resistance and glucose intolerance, indicating that PPARγ is required for maturation of alternatively activated macrophages (Odegaard et al. 2007). In the area of inflammation alone, PPARγ regulates not only the chemokine system, but also production of inflammatory cytokines (TNFα and IL-1β) (Jiang et al. 1998), matrix metalloproteinases (Shu et al. 2000), inducible nitric-oxide synthase (iNOS) (Ricote et al. 1998b), and expression of several adhesion molecules, including VCAM-1 and ICAM-1 (Pasceri et al. 2000), which may facilitate monocyte recruitment into the arterial wall.
PPARγ activation also induces uptake of oxLDL through transcriptional induction of the scavenger receptor CD36, thereby promoting macrophage foam cell formation. In addition to providing excess cellular cholesterol, internalized oxLDL upregulates PPARγ expression, thus promoting further PPARγ activation and CD36 upregulation (Tontonoz et al. 1998). Conditions of chronic lipid overload associated with a western diet would be predicted to stimulate these effects of PPARγ on CD36 and CX3CR1 expression and thus may explain why vasculoprotective effects of PPARγ agonists in treatment of atherosclerosis are moderately beneficial.
Results of genetic studies of PPARγ in man and in mouse models of disease are difficult to interpret in light of the effects of TZDs on the metabolic syndrome and atherosclerosis. The loss-of-function human PPARγ allele Pro12Ala causes decreased basal transcriptional activity following stimulation with synthetic PPARγ ligands (Masugi et al. 2000), yet has been associated with reduced risk of atherosclerotic cardiovascular disease (Ridker et al. 2003). Approximately 15% of Caucasians have this allele, which was originally associated with decreased risk of T2DM and lower body mass index (BMI) (Deeb et al. 1998). In contrast, Barroso et al (1999) reported in a small study that individuals heterozygous for loss-of-function mutations known as Val290Met or Pro467Leu that affect the ligand-binding domain of PPARγ exhibit marked insulin resistance with early onset of T2DM and hypertension.
Studies of potential PPARγ mechanisms in atherosclerosis have also been hindered by the fact that homozygous PPARγ deficiency is lethal in the mouse owing to placental dysfunction and myocardial thinning (Barak et al. 1999). PPARγ+/− mice are viable and have enhanced insulin sensitivity (Miles et al. 2000). Skeletal muscle-, adipocyte- and cardiomyocyte-specific disruption of PPARγ causes glucose intolerance and progressive insulin resistance in skeletal muscle (Hevener et al. 2003), decreased numbers of adipocytes and progressive lipodystrophy (He et al. 2003), and cardiac hypertrophy (Duan et al. 2005), respectively. Selective deletion of PPARγ in macrophages results in accelerated atherosclerosis and reduced basal cholesterol efflux (Akiyama et al. 2002). Similarly, conditional PPARγ−/−ldlr−/− mice fed an atherogenic diet have enhanced atherosclerosis compared to wild type controls (Babaev et al. 2005). Together, these results demonstrate an atheroprotective role of PPARγ in mouse.
Conclusions
Lipid components of the atheromatous microenvironment are major regulators of macrophage function in atherosclerosis (Witztum 1994). We have reviewed a novel oxidized lipid-activated pathway in macrophages involving activation of the transcription factor PPARγ and its ability to oppositely regulate expression of the major macrophage chemokine receptors CCR2 and CX3CR1 (Han et al. 2000, Barlic et al. 2006). Studies at the molecular, cellular, genetic and epidemiologic level provide evidence that blockade of CCR2 and/or CX3CR1 may be beneficial in atherosclerotic cardiovascular disease. PPARγ regulates many other genes and has pleotropic actions, so that its role in atherogenesis is complex, a balance of both pro-atherogenic and anti-atherogenic activities. However, preliminary clinical studies involving patients with T2DM suggest that PPARγ agonists may be beneficial in treatment of T2DM-driven cardiovascular disease (Home et al. 2007), perhaps in part through its regulation of macrophage CCR2 and CX3CR1.
Acknowledgments
The authors thank the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and the Wellcome Trust for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello RJ, Bourassa PA, Lindsey S, et al. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–1525. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- Akiyama TE, Sakai S, Lambert G, et al. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol. 2002;22:2607–2619. doi: 10.1128/MCB.22.8.2607-2619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaev VR, Yancey PG, Ryzhov SV, et al. Conditional knockout of macrophage PPAR{gamma}increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:1647–1653. doi: 10.1161/01.ATV.0000173413.31789.1a. [DOI] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Barlic J, Zhang Y, Foley JF, Murphy PM. Oxidized lipid-driven chemokine receptor switch, CCR2 to CX3CR1, mediates adhesion of human macrophages to coronary artery smooth muscle cells through a peroxisome proliferator-activated receptor {gamma}-dependent pathway. Circulation. 2006;114:807–819. doi: 10.1161/CIRCULATIONAHA.105.602359. [DOI] [PubMed] [Google Scholar]
- Barroso I, Gurnell M, Crowley VE, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- Campia U, Matuskey LA, Panza JA. Peroxisome proliferator-activated receptor-gamma activation with pioglitazone improves endothelium-dependent dilation in nondiabetic patients with major cardiovascular risk factors. Circulation. 2006;113:867–875. doi: 10.1161/CIRCULATIONAHA.105.549618. [DOI] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- Combadiere C, Potteaux S, Gao JL, et al. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107:1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- Daoudi M, Lavergne E, Garin A, et al. Enhanced adhesive capacities of the naturally occurring Ile249-Met280 variant of the chemokine receptor CX3CR1. J Biol Chem. 2004;279:19649–19657. doi: 10.1074/jbc.M313457200. [DOI] [PubMed] [Google Scholar]
- Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20:284–287. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- Fong AM, Robinson LA, Steeber DA, et al. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garton KJ, Gough PJ, Blobel CP, et al. Tumor necrosis factor-alpha -converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littmann DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- Gerrity RG, Goss JA, Soby L. Control of monocyte recruitment by chemotactic factor(s) in lesion-prone areas of swine aorta. Arteriosclerosis. 1985;5:55–66. doi: 10.1161/01.atv.5.1.55. [DOI] [PubMed] [Google Scholar]
- Ghilardi G, Biondi ML, Turri O, Guagnellini E, Scorza R. Internal carotid artery occlusive disease and polymorphisms of fractalkine receptor CX3CR1: a genetic risk factor. Stroke. 2004;35:1276–1279. doi: 10.1161/01.STR.0000128528.56009.d4. [DOI] [PubMed] [Google Scholar]
- Glavind J, Hartmann S. The occurrence of peroxidized lipids in atheromatous human aortas. Experimentia. 1951;7:464. doi: 10.1007/BF02168696. [DOI] [PubMed] [Google Scholar]
- Gosling J, Slaymaker S, Gu L, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein. B J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Okada Y, Clinton SK, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- Guo J, Van Eck M, Twisk J, et al. Transplantation of monocyte CC-chemokine receptor 2-deficient bone marrow into ApoE3-Leiden mice inhibits atherogenesis. Arterioscler Thromb Vasc Biol. 2003;23:447–453. doi: 10.1161/01.ATV.0000058431.78833.F5. [DOI] [PubMed] [Google Scholar]
- Han KH, Chang MK, Boullier A, et al. Oxidized LDL reduces monocyte CCR2 expression through pathways involving peroxisome proliferator-activated receptor {gamma} J Clin Invest. 2000;106:793–802. doi: 10.1172/JCI10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland WA, Gilbert JD, Brooks CJ. Lipids of human atheroma. 8. Oxidised derivatives of cholesteryl linoleate. Biochim Biophys Acta. 1973;316:378–385. [PubMed] [Google Scholar]
- He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevener AL, He W, Barak Y, et al. Muscle-specific PPARγ deletion causes insulin resistance. Nat Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes — an interim analysis. N Engl J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Jira W, Spiteller G, Carson W, Schramm A. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem Phys Lipid. 1998;91:1–11. doi: 10.1016/s0009-3084(97)00095-9. [DOI] [PubMed] [Google Scholar]
- Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-{gamma} calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev. 2004;25:899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Lei ZB, Zhang Z, Jing Q, et al. OxLDL upregulates CXCR2 expression in monocytes via scavenger receptors and activation of p38 mitogen-activated protein kinase. Cardiovasc Res. 2002;53:524–532. doi: 10.1016/s0008-6363(01)00491-6. [DOI] [PubMed] [Google Scholar]
- Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1-/- mice reveals a role for fractalkine in atherogenesis. J Clin Invest. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AC, Palinski W. Peroxisome proliferator-activated receptors: how their effects on macrophages can lead to the development of a new drug therapy against atherosclerosis. Annu Rev Pharmacol Toxicol. 2006;46:1–39. doi: 10.1146/annurev.pharmtox.46.120604.141247. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Weber C. Transmembrane chemokines: versatile ‘special agents’ in vascular inflammation. Thromb Haemost. 2007;97:649–703. [PubMed] [Google Scholar]
- Masugi J, Tamori Y, Mori H, Koike T, Kasuga M. Inhibitory effect of a proline-to-alanine substitution at codon 12 of peroxisome proliferator-activated receptor-gamma 2 on thiazolidinedione-induced adipogenesis. Biochem Biophys Res Commun. 2000;268:178–182. doi: 10.1006/bbrc.2000.2096. [DOI] [PubMed] [Google Scholar]
- McDermott DH, Fong AM, Yang Q, et al. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest. 2003;111:1241–1250. doi: 10.1172/JCI16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DH, Halcox JPJ, Schenke WH, et al. Association between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosis. Circ Res. 2001;89:401–407. doi: 10.1161/hh1701.095642. [DOI] [PubMed] [Google Scholar]
- McDermott DH, Yang Q, Kathiresan S, et al. CCL2 polymorphisms are associated with serum monocyte chemoattractant protein-1 levels and myocardial infarction in the Framingham Heart Study. Circulation. 2005;112:1113–1120. doi: 10.1161/CIRCULATIONAHA.105.543579. [DOI] [PubMed] [Google Scholar]
- Meisner F, Walcher D, Gizard F, et al. Effect of rosiglitazone treatment on plaque inflammation and collagen content in nondiabetic patients: data from a randomized placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2006;26:845–850. doi: 10.1161/01.ATV.0000203511.66681.7f. [DOI] [PubMed] [Google Scholar]
- Miles PD, Barak Y, He W, Evans RM, Olefsky JM. Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J Clin Invest. 2000;105:287–292. doi: 10.1172/JCI8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moatti D, Faure S, Fumeron F, et al. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001;97:1925–1928. doi: 10.1182/blood.v97.7.1925. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, et al. International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;88:1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Ortlepp JR, Vesper K, Mevissen V, et al. Chemokine receptor (CCR2) genotype is associated with myocardial infarction and heart failure in patients under 65 years of age. J Mol Med. 2003;81:363–367. doi: 10.1007/s00109-003-0435-x. [DOI] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzales RR, Goforth MH, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;446:1116–1121. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–238. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- Rayner K, Van ES, Groot PH, Reape TJ. Localisation of mRNA for JE/MCP-1 and its receptor CCR2 in atherosclerotic lesions of the ApoE knockout mouse. J Vasc Res. 2000;37:93–102. doi: 10.1159/000025720. [DOI] [PubMed] [Google Scholar]
- Ricote M, Huang J, Fajas L, et al. Expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1998a;95:7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998b;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR, Cheng S, Erlich HA, Lindpaintner K, Plutzky J, Zee RY. Alanine for proline substitution in the peroxisome proliferator-activated receptor gamma-2 (PPARG2) gene and the risk of incident myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:859–863. doi: 10.1161/01.ATV.0000068680.19521.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Vazquez R, Marzoa-Rivas R, Gil-Ortega I, Kaski JC. Peroxisome proliferator-activated receptor-gamma agonists for management and prevention of vascular disease in patients with and without diabetes mellitus. Am J Cardiovasc Drugs. 2006;6:231–242. doi: 10.2165/00129784-200606040-00003. [DOI] [PubMed] [Google Scholar]
- Ristow M, Muller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med. 1998;339:953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis -- an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Shu H, Wong B, Zhou G, Li Y, Berger J, Woods JW, Wright SD, Cai TQ. Activation of PPARalpha or gamma reduces secretion of matrix metalloproteinase 9 but not interleukin 8 from human monocytic THP-1 cells. Biochem Biophys Res Commun. 2000;267:345–349. doi: 10.1006/bbrc.1999.1968. [DOI] [PubMed] [Google Scholar]
- Sidhu JS, Cowan D, Kaski JC. The effects of rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, on markers of endothelial cell activation, C-reactive protein, and fibrinogen levels in non-diabetic coronary artery disease patients. J Am Coll Cardiol. 2003;42:1757–1763. doi: 10.1016/j.jacc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Szalai C, Duba J, Prohaszka Z, Kalina A, Szabo T, Nagy B, Horvath L, Csaszar A. Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD). Coincidence of elevated Lp(a) and MCP-1 -2518 G/G genotype in CAD patients. Atherosclerosis. 2001;158:233–239. doi: 10.1016/s0021-9150(01)00423-3. [DOI] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teupser D, Pavlides S, Tan M, Gutierrez-Ramos JC, Kolbeck R, Breslow JL. Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proc Natl Acad Sci U S A. 2004;101:17795–17800. doi: 10.1073/pnas.0408096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- Verreth W, Ganame J, Mertens A, Bernar H, Herregods MC, Holvoet P. Peroxisome proliferator-activated receptor-{alpha}, {gamma}-agonist improves insulin sensitivity and prevents loss of left ventricular function in obese dyslipidemic mice. arterioscler thromb vasc biol. 2006;26:922–928. doi: 10.1161/01.ATV.0000207318.42066.bb. [DOI] [PubMed] [Google Scholar]
- Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet. 1994;344:793–795. doi: 10.1016/s0140-6736(94)92346-9. [DOI] [PubMed] [Google Scholar]
- Weber C, Schober A, Zernecke A. Chemokines - key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Atherioscler Thromb Vasc Biol. 2004;24:1997–2008. doi: 10.1161/01.ATV.0000142812.03840.6f. [DOI] [PubMed] [Google Scholar]
- Wong BWC, Wong D, McManus BM. Characterization of fractalkine (CX3CL1) and CX3CR1 in human coronary arteries with native atherosclerosis, diabetes mellitus, and transplant vascular disease. Cardiovasc Pathol. 2002;11:332–338. doi: 10.1016/s1054-8807(02)00111-4. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S, Lipton BA, Rosenfeld ME, et al. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991;88:5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]


