Abstract
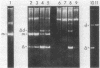
Certain pBR322-related plasmids containing direct repeats of the insertion element IS50 appear to be unstable in recA Escherichia coli because smaller recombinant derivatives accumulate rapidly in plasmid DNA populations. We show here that (i) this instability is plasmid specific, but not IS50 specific; (ii) it is due to a detrimental effect exerted by these plasmids on bacterial growth; and (iii) the growth impairment is alleviated in cells harboring the smaller recombinant plasmids. Although a recent report had concluded that accumulation of recombinants reflected an IS50-specific recombination function, when correction is made for the relative growth rates of cells containing the parental and recombinant plasmids the evidence for such a recombination function disappears.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur A., Sherratt D. Dissection of the transposition process: a transposon-encoded site-specific recombination system. Mol Gen Genet. 1979 Oct 1;175(3):267–274. doi: 10.1007/BF00397226. [DOI] [PubMed] [Google Scholar]
- Auerswald E. A., Ludwig G., Schaller H. Structural analysis of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):107–113. doi: 10.1101/sqb.1981.045.01.019. [DOI] [PubMed] [Google Scholar]
- Berg C. M. Auxotroph accumulation in deoxyribonucleic acid polymeraseless strains of Escherichia coli K-12. J Bacteriol. 1971 Jun;106(3):797–801. doi: 10.1128/jb.106.3.797-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Johnsrud L., McDivitt L., Ramabhadran R., Hirschel B. J. Inverted repeats of Tn5 are transposable elements. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2632–2635. doi: 10.1073/pnas.79.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E. Structural requirement for IS50-mediated gene transposition. Proc Natl Acad Sci U S A. 1983 Feb;80(3):792–796. doi: 10.1073/pnas.80.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek D., Roth J. R. Regulation of Tn5 transposition in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6047–6051. doi: 10.1073/pnas.77.10.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel S. W., Berg D. E. Mechanism of IS1 transposition in E. coli: choice between simple insertion and cointegration. Genetics. 1984 Oct;108(2):319–330. doi: 10.1093/genetics/108.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Doherty M. J., Morrison P. T., Kolodner R. Genetic recombination of bacterial plasmid DNA. Physical and genetic analysis of the products of plasmid recombination in Escherichia coli. J Mol Biol. 1983 Jul 5;167(3):539–560. doi: 10.1016/s0022-2836(83)80097-7. [DOI] [PubMed] [Google Scholar]
- Dykhuizen D. E., Hartl D. L. Selection in chemostats. Microbiol Rev. 1983 Jun;47(2):150–168. doi: 10.1128/mr.47.2.150-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel R. A., James A. A., Kolodner R. recA-independent general genetic recombination of plasmids. Nature. 1981 Nov 12;294(5837):184–186. doi: 10.1038/294184a0. [DOI] [PubMed] [Google Scholar]
- Gill R., Heffron F., Dougan G., Falkow S. Analysis of sequences transposed by complementation of two classes of transposition-deficient mutants of Tn3. J Bacteriol. 1978 Nov;136(2):742–756. doi: 10.1128/jb.136.2.742-756.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschel B. J., Berg D. E. A derivative of Tn5 with direct terminal repeats can transpose. J Mol Biol. 1982 Feb 25;155(2):105–120. doi: 10.1016/0022-2836(82)90439-9. [DOI] [PubMed] [Google Scholar]
- Hirschel B. J., Galas D. J., Berg D. E., Chandler M. Structure and stability of transposon 5-mediated cointegrates. J Mol Biol. 1982 Aug 25;159(4):557–580. doi: 10.1016/0022-2836(82)90101-2. [DOI] [PubMed] [Google Scholar]
- Meyer R., Boch G., Shapiro J. Transposition of DNA inserted into deletions of the Tn5 kanamycin resistance element. Mol Gen Genet. 1979 Mar 9;171(1):7–13. doi: 10.1007/BF00274009. [DOI] [PubMed] [Google Scholar]
- Reznikoff W. S. Tn5 transposition and its regulation. Cell. 1982 Dec;31(2 Pt 1):307–308. doi: 10.1016/0092-8674(82)90123-4. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1933–1937. doi: 10.1073/pnas.76.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupancic T. J., Marvo S. L., Chung J. H., Peralta E. G., Jaskunas S. R. RecA-independent recombination between direct repeats of IS50. Cell. 1983 Jun;33(2):629–637. doi: 10.1016/0092-8674(83)90444-0. [DOI] [PubMed] [Google Scholar]