Abstract
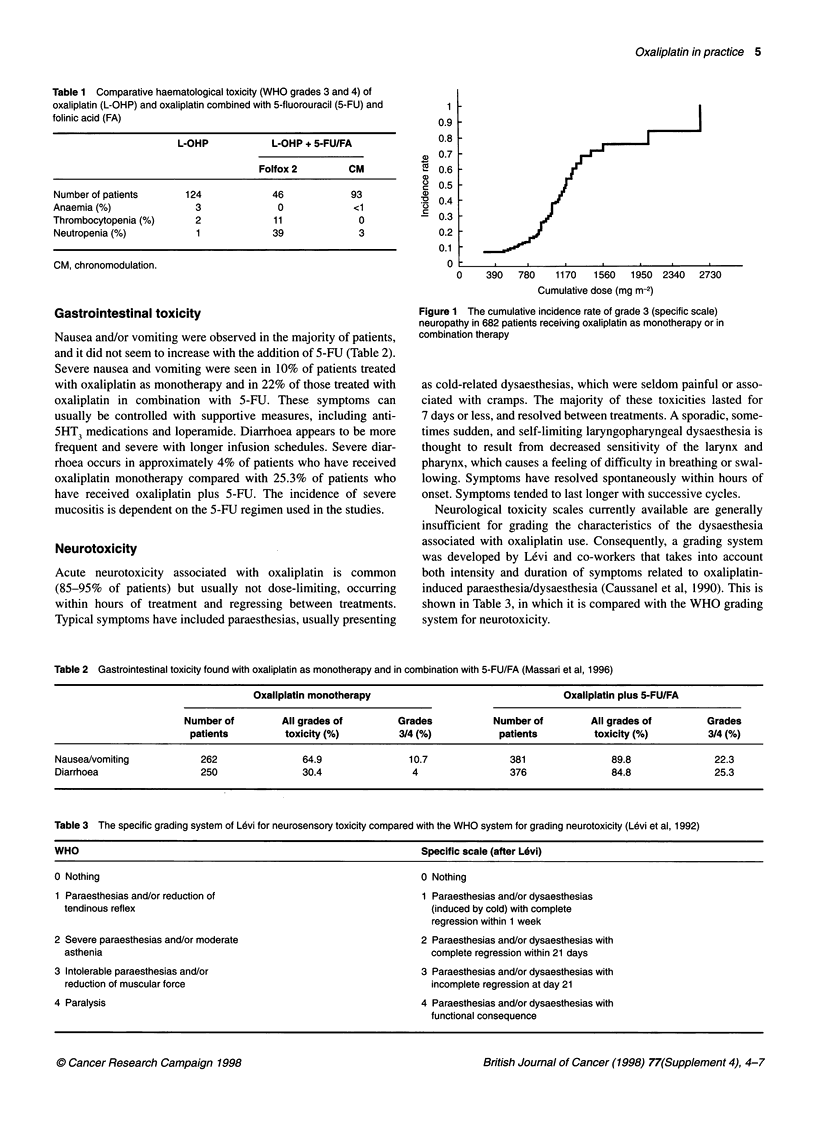
Oxaliplatin, a new third-generation platinum complex, is active in the treatment of colorectal and advanced ovarian cancers, both as monotherapy and in combination therapy. It has demonstrated a very good safety profile, characterized by low haematotoxicity, and moderate and manageable gastrointestinal toxicity. No significant renal or ototoxicities have been observed. Oxaliplatin induces a peripheral sensory neuropathy which is characterized by distal and perioral dysaesthesia, and is induced or exacerbated by the cold; in general, it is regressive between cycles of treatment. This dose-limiting toxicity is cumulative, but reversible within a few months of discontinuation of treatment in the majority of cases. In a cohort study of 490 patients with advanced colorectal cancer included in an extended access programme, more than 2700 cycles of oxaliplatin plus 5-fluorouracil (5-FU) were administered. The overall safety profile of oxaliplatin was shown to be very favourable. Oxaliplatin and cisplatin, each in combination with cyclophosphamide, have a similar efficacy in the treatment of advanced ovarian cancer, but oxaliplatin was better tolerated than cisplatin in terms of haematological, gastrointestinal, neurosensory and renal toxicities. The safety profile of oxaliplatin makes it an ideal candidate for combination therapy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caussanel J. P., Lévi F., Brienza S., Misset J. L., Itzhaki M., Adam R., Milano G., Hecquet B., Mathé G. Phase I trial of 5-day continuous venous infusion of oxaliplatin at circadian rhythm-modulated rate compared with constant rate. J Natl Cancer Inst. 1990 Jun 20;82(12):1046–1050. doi: 10.1093/jnci/82.12.1046. [DOI] [PubMed] [Google Scholar]
- Extra J. M., Espie M., Calvo F., Ferme C., Mignot L., Marty M. Phase I study of oxaliplatin in patients with advanced cancer. Cancer Chemother Pharmacol. 1990;25(4):299–303. doi: 10.1007/BF00684890. [DOI] [PubMed] [Google Scholar]
- Lévi F., Misset J. L., Brienza S., Adam R., Metzger G., Itzakhi M., Caussanel J. P., Kunstlinger F., Lecouturier S., Descorps-Declère A. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumor effectiveness against metastatic colorectal cancer. Cancer. 1992 Feb 15;69(4):893–900. doi: 10.1002/1097-0142(19920215)69:4<893::aid-cncr2820690410>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Mathé G., Kidani Y., Triana K., Brienza S., Ribaud P., Goldschmidt E., Ecstein E., Despax R., Musset M., Misset J. L. A phase I trial of trans-1-diaminocyclohexane oxalato-platinum (l-OHP). Biomed Pharmacother. 1986;40(10):372–376. [PubMed] [Google Scholar]
- Pendyala L., Creaven P. J. In vitro cytotoxicity, protein binding, red blood cell partitioning, and biotransformation of oxaliplatin. Cancer Res. 1993 Dec 15;53(24):5970–5976. [PubMed] [Google Scholar]
- Soulié P., Bensmaïne A., Garrino C., Chollet P., Brain E., Fereres M., Jasmin C., Musset M., Misset J. L., Cvitkovic E. Oxaliplatin/cisplatin (L-OHP/CDDP) combination in heavily pretreated ovarian cancer. Eur J Cancer. 1997 Aug;33(9):1400–1406. doi: 10.1016/s0959-8049(97)00122-6. [DOI] [PubMed] [Google Scholar]
- de Gramont A., Vignoud J., Tournigand C., Louvet C., André T., Varette C., Raymond E., Moreau S., Le Bail N., Krulik M. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer. 1997 Feb;33(2):214–219. doi: 10.1016/s0959-8049(96)00370-x. [DOI] [PubMed] [Google Scholar]


