Abstract
Although many vertebrates can synthesize ascorbic acid (vitamin C), it is still unclear from the evolutionary perspective when the ability to synthesize the vitamin first appeared in the animal kingdom and how frequently the trait has been lost. We report here ascorbic acid biosynthesis ability in sea lamprey (Petromyzon marinus) which represent the most ancient vertebrate lineage examined thus far for presence of gulonolactone oxidase, the enzyme catalyzing the terminal step in biosynthesis of vitamin C. This finding supports the view that the ancestors of living vertebrates were not scurvy prone and that the loss of gulonolactone oxidase activity subsequently occurred several times in vertebrate phylogeny. Adult sea lamprey allocate significant amounts of ascorbic acid to the gonads to guaranty high-quality gametes. Tissue stores of ascorbate were maintained by de novo synthesis (1.2–1.3 mg of ascorbic acid/300-g sea lamprey per day at 15°C) while sea lamprey fast during spawning migration. We estimate that the in vivo daily renewal rate of ascorbate is 4–5% of the whole-body ascorbate pool based on measurement of its biosynthesis and concentration in the whole animal.
Since the discovery of its function in scurvy-prone humans and guinea pigs in 1932 (1), l-ascorbic acid (AA, vitamin C) has attracted research in the areas of nutrition, aging, cancer ethiology (2), UV-B skin damage (3), and hormonal regulation (4). Less attention has been given to the evolution of AA synthesis in vertebrates. It is unclear when the ability to synthesize AA first appeared in the animal kingdom and how frequently the trait has been lost (5–7). We previously described gulonolactone oxidase (GLO, EC 1.1.3.8) activity in Chondrostei fishes (sturgeon and paddlefish) (8–10), the enzyme responsible for the oxidation of l-gulonolactone to AA (11). The general view is that all animals lacking GLO activity require an exogenous source of AA (12), whereas it is assumed that animals producing AA do not need an AA supply in their diet to achieve optimal growth and accomplish successful reproduction.
AA is an essential molecule in the overall health of humans and animals, including growth, bone development, immune system, and fertility. The roles of AA in reproduction are to participate in the biosynthesis of collagen, to take part in the biosynthesis of steroid and peptide hormones, and to prevent the oxidation of biomolecules. Specific transport systems for dehydroascorbic acid (oxidized vitamin C) have been identified in oocytes (13) in which AA accumulates against a concentration gradient, indicating a specific requirement of vitamin C in the gametes. In scurvy-prone teleost fishes, sperm concentration and motility were reduced by dietary AA deficiency (14). Fecundity and embryo survival both increased with dietary AA levels in rainbow trout (Oncorhynchus mykiss) females (15). The sea lamprey (Petromyzon marinus, Agnatha) represents an excellent model for reproductive physiologists because maturing sea lamprey allocate essential nutrients into the gonads while fasting, reach sexual maturity, and die shortly after spawning.
Adult landlocked sea lamprey enter rivers and streams tributary to the Great Lakes and migrate to the spawning grounds where reproduction occurs in the spring and summer. When migration starts, sea lamprey stop feeding and begin to mature. The cessation of feeding is associated with progressive atrophy of the intestine (16). Gonadal development during this period depends on fat and protein reserves mainly in muscles and skin.
This investigation was conducted (i) to examine whether sea lamprey, a phylogenetically ancient vertebrate, can synthesize AA de novo and (ii) to quantify AA allocation to specific tissues during reproduction. Kinetics data of fish GLO were used to project a daily rate of AA synthesis in upstream-migrating sea lamprey.
MATERIALS AND METHODS
Adult sea lamprey used in this investigation originated from Lake Erie. Upstream-migrating sea lamprey were trapped in May-June 1995 and 1996 at Cattaraugus Creek, Springville, NY (water temperature; 13–15°C). Sea lamprey weighed 289 ± 40 g (n = 6) in 1995 and 340 ± 41 g (n = 8) in 1996. Female sea lamprey were 5% heavier than males in both years.
GLO activity was measured in the kidneys and livers of each male (three in 1995 and four in 1996) and each female (three in 1995 and four in 1996) sea lamprey using the direct spectrophotometric assay (17). Kidneys and livers were removed, frozen in liquid nitrogen, and stored at −81°C until analyzed. Tissue samples were homogenized in 0.25 M sucrose (1 g of tissue in 5 ml of sucrose) at low speed for two periods of 10 sec using a spin homogenizer Omni 5000 (Omni International, Waterbury, CT). Homogenates were centrifuged at 15,000 × g for 15 min at 4°C. Pellets were discarded and supernatants were centrifuged at 100,000 × g for 60 min at 4°C. The pellets (or microsomes) were resuspended with 1 ml of 20 mM Tris-acetate (pH 8.0) containing 10 mM KCl, 1 mM EDTA, and 0.2% (wt/vol) sodium deoxycholate using a glass rod and a spin homogenizer (Omni 5000). The suspensions were then centrifuged at 14,000 × g for 35 min at 4°C to solubilize the microsomes and release the enzyme into solution. The top fraction was assayed at 25°C for GLO as the microsomal fraction of the tissue preparation. Protein content of the microsomal fraction was determined with the Bradford procedure (18) using commercial Coomassie protein assay reagent (Pierce). BSA was used as the standard. GLO activity was expressed as micrograms of AA formed per milligrams of microsomal protein per hour at 25°C. The rate of AA biosynthesis in vivo per sea lamprey per day was calculated at 15°C following the procedure used previously (19). The in vitro Michaelis constant (Km) of white sturgeon GLO for l-gulonolactone (0.92 mM; R.M., K.D., and P. H. Sato, submitted for publication) was used for calculation. Since the concentration of l-gulonolactone in our enzyme assay (10 mM) was 10-fold higher than Km, Michaelis-Menten kinetics were applied with the assumption that the measured velocities were close to the estimates of Vmax. The concentration of l-gulonolactone (S) in renal cells of sea lamprey was assumed to be no greater than 0.05 mM (19). A theoretical observed rate (V0) at 25°C was calculated for male and female sea lamprey by substituting into the equation V0 = Vmax⋅S/Km + S. The values were then multiplied by 6 (since 1 g of sea lamprey kidney contains approximately 6 mg of microsomal protein), followed by times 24 (to convert to 1 day), then times 2 (weight in grams of the kidneys in a 300-g male or female sea lamprey), and divided by 1.73 (Q10 between 15 and 25°C as determined for sea lamprey GLO) to express the rate of synthesis at 15°C (water temperature at Cattaraugus Creek).
Total and oxidized AA concentrations in liver, kidney, and testis of each male (three in 1995 and four in 1996) and in liver, kidney, and eggs of each female (three in 1995 and four in 1996) were determined using the 2,4-dinitrophenylhydrazine colorimetric method (20). The sum of reduced and oxidized forms of AA represents the total AA concentration. Tissues were homogenized in 5% (wt/vol) trichloroacetic acid solution containing 250 mM HClO4 and 0.08% (wt/vol) EDTA using a spin homogenizer (Omni 5000) and centrifuged at 29,000 × g for 30 min at 4°C. Supernatants were stored at −81°C until assayed.
Total AA concentration in the whole body minus liver, kidney, and gonads was determined using HPLC with an electrochemical detection (HPLC-EC) procedure from three males and three females sampled in 1996. The HPLC system comprised a 25-cm × 4.6-mm, 5-μm particle size, PLRP-S column (Polymer Laboratories, Amherst, MA), an amperometric detector LC-4C (Bioanalytical Systems, West Lafayette, IN), and a cross-flow cell equipped with a glass carbon electrode and an Ag/AgCl reference electrode. The applied potential was +0.575 V (oxidative mode). The mobile phase consisted of 20 mM NaH2PO4⋅H2O, 0.06% (wt/vol) metaphosphoric acid, and 0.4% (vol/vol) acetonitrile (pH 3.0) delivered at a flow rate of 0.4 ml/min. Samples were homogenized (1 g of tissue in 10 ml) in cold 0.1% (wt/vol) metaphosphoric acid containing 0.1 mM EDTA and 1 mM thiourea (pH 2.2), and centrifuged at 29,000 × g for 30 min at 4°C. The supernatants were kept at −81°C until assayed. One milliliter of supernatant was incubated with 1 ml of 1% (wt/vol) dl-homocysteine and 8 ml of 50 mM sodium phosphate buffer (pH 7.2) containing 0.1 mM EDTA and 1 mM thiourea for 30 min at room temperature. The reaction was stopped by transferring 0.5 ml of incubate into 0.5 ml of cold 3.5% (wt/vol) metaphosphoric acid. The samples were filtered through a 0.45-μm nylon membrane prior to injection.
Statistical analysis was performed using the unpaired Student’s t test. The accepted level of significance was 0.05.
RESULTS AND DISCUSSION
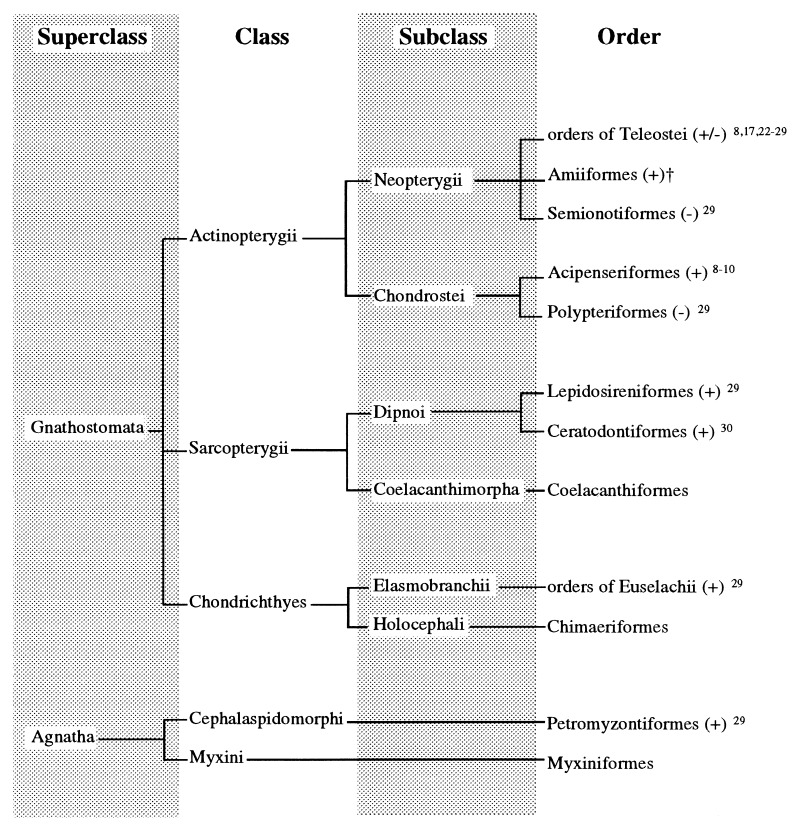
Lamprey (Agnatha, Petromyzontiformes) belong to the most phylogenetically ancient group of fishes (origin of Petromyzontiformes 420 million yr before the present; ref. 21) ever studied regarding AA biosynthesis (Fig. 1). GLO activity was found in the kidney of sea lamprey and was associated with the microsomal fraction. The levels of enzyme activity (126–159 μg AA/mg microsomal protein per h at 25°C) were not significantly different (P > 0.05) irrespective of the sex or the year of sampling (Table 1). No enzyme activity was detected in the liver. GLO activity is reported in the kidney of landlocked sea lamprey (P. marinus), corroborating previous data of trace activity in Lampetra japonica (29). The present study supports the notion that kidneys are the site of AA biosynthesis in poikilothermic vertebrates such as amphibians and reptiles (31, 32), Dipnoi (29, 30), and Chondrostei fishes (8–10) and extends this view to a representative of jawless vertebrates (Agnatha). Since there is no instance of re-acquisition of GLO activity once lost, present evidence suggests that the common ancestor of Agnatha and Gnathostomata, that existed in the Cambrian period (590–500 million yr before present), possessed GLO from which living aquatic and terrestrial vertebrates evolved. Independent episodes of evolution led to the loss of GLO activity (due to missense mutations in the coding region of the gene; refs. 33 and 34) several times in vertebrate phylogeny, such as in teleost fishes (17), passeriform birds (6), bats (7), guinea pigs (5, 33), and primates (5, 34).
Figure 1.
Hierarchy of living fishes. A + denotes that at least one species in a given order was reported to biosynthesize AA de novo due to the presence of GLO, whereas − denotes the incapability as the result of the loss of the enzyme activity. †, R.M. and K.D., unpublished data. (Adapted from ref. 38).
Table 1.
AA biosynthesis in the kidney of 300-g adult sea lampreys
| Year | Male | Female | |
|---|---|---|---|
| Gulonolactone oxidase activity | |||
| (μg AA/ mg microsomal protein⋅h | 1995 | 126 ± 17 | 159 ± 62 |
| at 25°C) | 1996 | 142 ± 17 | 150 ± 36 |
| Rate of AA biosynthesis | |||
| (mg AA/sea lamprey⋅day at 15°C) | — | 1.2 | 1.3 |
Mean ± SD of three to four males or females. Gulonolactone oxidase activity was not significantly different (P > 0.05) between sexes in 1995 or in 1996. Likewise, the enzyme activity was not significantly different among males or among females in 1995 compared to 1996.
Parasitic sea lamprey rely on endogenously produced AA to maintain the AA pool in their reproductive organs. Because blood plasma of fish fed upon by lamprey has a low concentration of AA (1–5 μg /ml) (35), dietary AA is likely to be a marginal source compared with AA produced enzymatically in the kidneys. When examined in relation to terrestrial mammals, the calculated rate of AA synthesis in the 300-g sea lamprey (1.2–1.3 mg AA/300-g sea lamprey per day at 15°C) was found to be intermediate to that of the 350-g rat (5.4 mg AA/liver per day at 37°C) and that of the 25-g mouse (0.3 mg AA/liver per day at 37°C) (19). Also, upstream-migrating sea lamprey do not feed and depend exclusively on endogenously produced vitamin C to compensate for metabolized AA. Thus, the synthesis rate can be viewed as a reliable estimate of the recommended daily allowance for AA maintenance in migrating lamprey. In vivo studies using 220- to 347-g male rats and i.p. 14C-labeled AA administration as the sole exogenous supply of vitamin C showed that the amount of AA synthesized daily (2.6 mg AA/100 g body weight) was about the same as the amount required each day in the diet of guinea pigs of similar body weight to maintain tissue saturation (36). Assuming that migrating sea lamprey are in a steady state in respect to AA synthesis and degradation and consequently the body pool of AA remains constant during the upstream migration, the synthesis rate corresponds to the turnover rate, i.e., the amount of body AA renewed per day. Based on our calculations (daily synthesis/whole-body pool, percent), approximately 4–5% of the whole-body AA pool is renewed daily via biosynthesis in 300-g sea lamprey at 15°C irrespective of the sex. For comparison, the whole-body pool of AA in 270- to 355-g adult male rats amounted to 24–43 mg of AA and approximately 5–8 mg of AA was synthesized per day (37). Thus, the turnover rate of the body pool per day in the rat ranged from 19 to 21%.
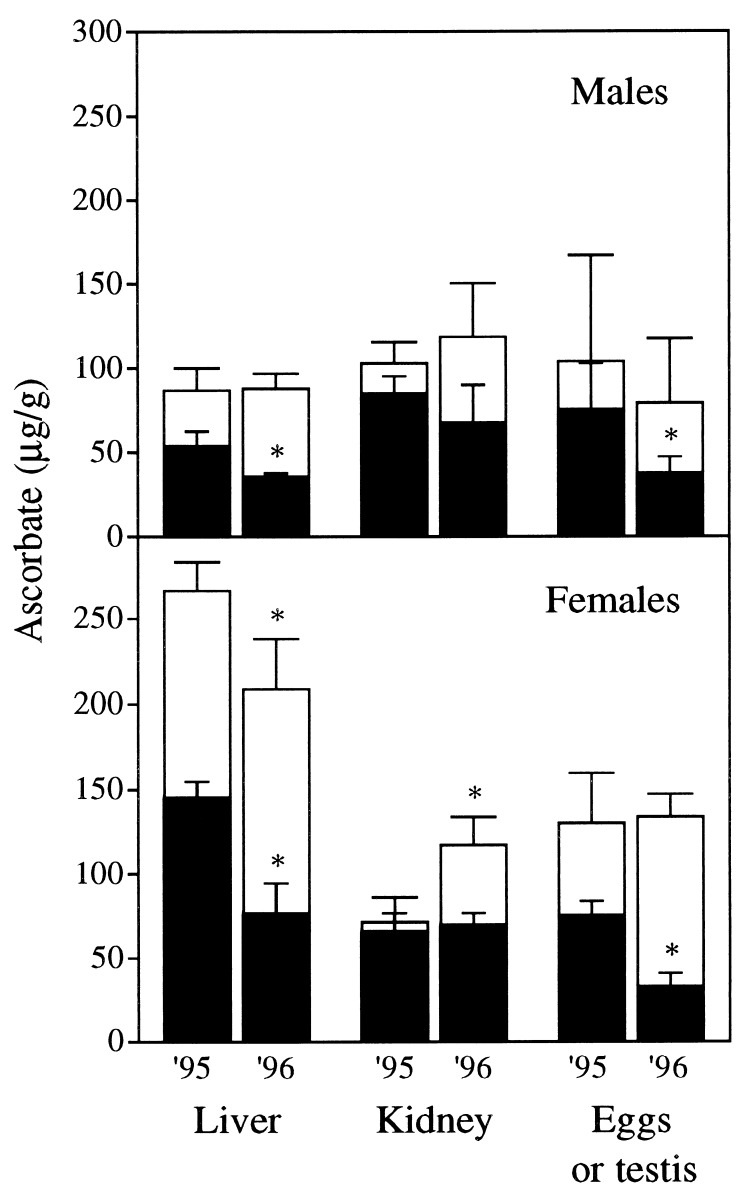
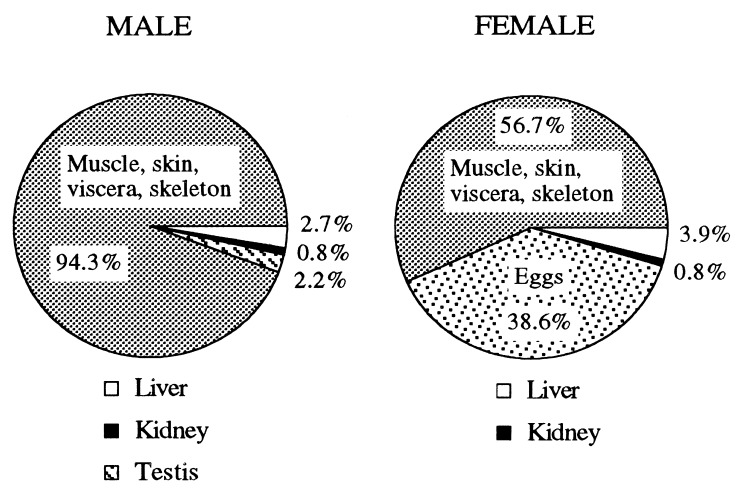
AA allocation was influenced by sea lamprey sex. In males, tissue total AA concentrations did not differ significantly between 1995 and 1996 (Fig. 2). The oxidized AA concentrations in the liver and testis were significantly (P < 0.05) lower in males sampled in 1996 as compared with those sampled in 1995. Large variations among individuals were observed in the testes. Most of the AA found in the liver, kidney, and testis was in the oxidized state. Female sea lamprey sampled in 1995 differed significantly (P < 0.05) from those sampled in 1996 with respect to liver AA concentrations. Significant differences were also found in kidney total AA and egg oxidized AA concentrations in female sea lamprey. In the livers of females, total AA concentrations were three times higher than in males. Liver, kidney, and gonad represented a small portion (5.7%) of the total AA in males, whereas the same three tissues accounted for 43.3% of the total AA in females (Fig. 3). The disparity was related to a much higher gonadosomatic index in females and a concomitantly lower concentration of total AA in testes (79.3–104.1 μg/g) than in eggs (130–133.8 μg/g). Whole-body total AA was not significantly different between females and males: 28.8 ± 1.2 vs. 26.7 ± 1.6 mg AA/300-g sea lamprey, respectively. The greater content of AA in eggs than in testes was balanced by the lower AA concentration in the remainder of the females (once liver, kidney, and gonads were removed): 16.4 ± 1.8 mg AA/individual compared with male bodies; 25.2 ± 1.7 mg AA/individual. Total AA concentrations in muscle tissue and notochord averaged 40 μg/g in both male and female sea lamprey. Total AA concentrations in sea lamprey skin were the highest among the tissues analyzed and differed markedly between sexes: 360 μg/g in females versus 460 μg/g in males. Further study is required to explain the role of the skin as a storage site of vitamin C, possibly in osmoregulation and body defenses.
Figure 2.
Total AA (whole columns) and oxidized AA (black columns) concentrations in liver, kidney, and gonads of male (Top) and female (Bottom) sea lampreys trapped in 1995 and 1996 during the upstream migration to the spawning grounds. Mean ± SD of three to four males or females. An ∗ denotes that total AA or oxidized AA concentration in a given tissue was significantly different in 1996 compared with 1995 (P < 0.05). The hepatosomatic indices (liver weight/body weight, percent) were higher in males [2.6 ± 0.4% (n = 7)] than in females [1.4 ± 0.3% (n = 7)] in 2 subsequent yr. The gonadosomatic index (gonad weight/body weight, percent) was 1.9 ± 0.5% (n = 5) in males sampled in 1995 and 1996. Female gonadosomatic index was 25.0 ± 4.8% (n = 7). The relative weight of kidney was 0.68 ± 0.04% (n = 14) of total body weight irrespective of the year of sampling or the sex.
Figure 3.
Total AA distribution in adult sea lampreys trapped during the upstream migration to the spawning grounds. Data collected in 1995 and 1996 were combined for calculations. Proportions are expressed as percentage of total body pool.
Upstream-migrating sea lamprey studied in 1995 and 1996 displayed similar AA concentrations in their tissues, indicating that males and females had consistent AA status in both years while approaching the spawning grounds. Male and female sea lamprey also showed consistency in whole-body AA content and rate of AA biosynthesis. However, AA was differently distributed within the body depending on the sex. AA deposition in eggs exceeded that of any other organs, representing 38.6% of the total pool of AA in female sea lampreys. In contrast, testis AA amounted to 2.2% of total body pool in males. Male and female sea lamprey showed comparable levels of GLO activity, indicating that discrepancies regarding the tissue AA allocation between sexes were the result of distinct patterns of distribution within tissues rather than different rates of AA biosynthesis. It also suggests that AA deposited into the gonads was transferred from reserve tissues such as the muscle and the skin, transported via the blood, and accumulated against a concentration gradient into the gametes requiring specific transporters (13).
The process of AA accumulation into sea lamprey eggs is in agreement with previous studies conducted in scurvy-prone teleost fishes (14, 15). This results in high concentrations of AA and stresses the importance of AA in gonadogenesis of all vertebrates irrespective of the ability to biosynthesize vitamin C.
Acknowledgments
We thank Dr. A. Ciereszko for assistance with sea lamprey sampling and Drs. F. W. H. Beamish and J. H. Youson for critical reading of the draft. This study was partly funded by the Great Lakes Fishery Commission (Ann Arbor, MI) and the Ohio Sea Grant College Program. Salaries were partly provided by state and federal funds appropriated to the Ohio Agriculture Research and Development Center (Wooster, OH).
ABBREVIATIONS
- GLO
l-gulono-1,4-lactone oxidase (EC 1.1.3.8)
- AA
ascorbic acid
References
- 1.Svirbely J L, Szent-Györgyi A. Nature (London) 1932;129:576–577. [Google Scholar]
- 2.Weber P, Bendich A, Schalach W. Int J Vitam Nutr Res. 1996;66:19–30. [PubMed] [Google Scholar]
- 3.Kobayashi S, Takehana M, Itoh S, Ogata E. Photochem Photobiol. 1996;64:224–228. doi: 10.1111/j.1751-1097.1996.tb02447.x. [DOI] [PubMed] [Google Scholar]
- 4.Wells W W, Dou C-Z, Dybas L N, Jung C-H, Kalbach H L, Xu D P. Proc Natl Acad Sci USA. 1995;92:11869–11873. doi: 10.1073/pnas.92.25.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns J J. Nature (London) 1957;180:553. doi: 10.1038/180553a0. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhuri C R, Chatterjee I B. Science. 1969;164:435–436. doi: 10.1126/science.164.3878.435. [DOI] [PubMed] [Google Scholar]
- 7.Birney E C, Jenness R, Ayaz K M. Nature (London) 1976;260:626–628. doi: 10.1038/260626a0. [DOI] [PubMed] [Google Scholar]
- 8.Moreau R, Dabrowski K. J Comp Physiol B. 1996;166:178–183. [Google Scholar]
- 9.Dabrowski D. Experientia. 1994;50:745–748. [Google Scholar]
- 10.Moreau R, Kaushik S J, Dabrowski K. Fish Physiol Biochem. 1996;15:431–438. doi: 10.1007/BF01875586. [DOI] [PubMed] [Google Scholar]
- 11.Hassan M, Lehninger A L. J Biol Chem. 1956;223:123–128. [PubMed] [Google Scholar]
- 12.Sato P, Nishikimi M, Udenfriend S. Biochem Biophys Res Commun. 1976;71:293–299. doi: 10.1016/0006-291x(76)90281-3. [DOI] [PubMed] [Google Scholar]
- 13.Vera J C, Rivas C I, Fischbarg J, Golde D W. Nature (London) 1993;364:79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- 14.Ciereszko A, Dabrowski K. Biol Reprod. 1995;52:982–988. doi: 10.1095/biolreprod52.5.982. [DOI] [PubMed] [Google Scholar]
- 15.Blom J H, Dabrowski K. Biol Reprod. 1995;52:1073–1080. doi: 10.1095/biolreprod52.5.1073. [DOI] [PubMed] [Google Scholar]
- 16.Vladykov V D, Mukerji G N. J Fish Res Board Can. 1961;18:1125–1143. [Google Scholar]
- 17.Dabrowski K. Biol Chem Hoppe-Seyler. 1990;371:207–214. doi: 10.1515/bchm3.1990.371.1.207. [DOI] [PubMed] [Google Scholar]
- 18.Bradford M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Rucker R B, Dubick M A, Mouritsen J. Am J Clin Nutr. 1980;33:961–964. doi: 10.1093/ajcn/33.5.961. [DOI] [PubMed] [Google Scholar]
- 20.Dabrowski K, Hinterleitner S. Analyst. 1989;114:83–87. doi: 10.1039/an9891400083. [DOI] [PubMed] [Google Scholar]
- 21.Goodman M, Miyamoto M M, Czelusniak J. In: Molecules and Morphology in Evolution: Conflict or Compromise? Patterson C, editor. Cambridge, U.K.: Cambridge Univ. Press; 1987. pp. 141–176. [Google Scholar]
- 22.Chatterjee I B. Sci Cult. 1973;39:210–212. [Google Scholar]
- 23.Wilson R P. Comp Biochem Physiol. 1973;46B:635–638. doi: 10.1016/0305-0491(73)90103-x. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto Y, Sato M, Ikeda S. Bull Jpn Soc Sci Fish. 1978;44:775–779. [Google Scholar]
- 25.Sato M, Yoshinaka R, Yamamoto Y, Ikeda S. Bull Jpn Soc Sci Fish. 1978;44:1151–1156. [Google Scholar]
- 26.Soliman A K, Jauncey K, Roberts R J. Aquacult Fish Manag. 1985;16:249–256. [Google Scholar]
- 27.Thomas P, Bally M B, Neff J M. J Fish Biol. 1985;27:47–57. [Google Scholar]
- 28.Dabrowski K. Fish Physiol Biochem. 1991;9:215–221. doi: 10.1007/BF02265142. [DOI] [PubMed] [Google Scholar]
- 29.Touhata K, Toyohara H, Mitani T, Kinoshita M, Satou M, Sakaguchi M. Fish Sci. 1995;61:729–730. [Google Scholar]
- 30.Dykhuizen D E, Harrison K M, Richardson B J. Experientia. 1980;36:945–946. doi: 10.1007/BF01953807. [DOI] [PubMed] [Google Scholar]
- 31.Roy R N, Guha B C. Nature (London) 1958;182:319–320. doi: 10.1038/182319a0. [DOI] [PubMed] [Google Scholar]
- 32.Chatterjee I B. Science. 1973;182:1271–1272. doi: 10.1126/science.182.4118.1271. [DOI] [PubMed] [Google Scholar]
- 33.Nishikimi M, Kawai T, Yagi K. J Biol Chem. 1992;267:21967–21972. [PubMed] [Google Scholar]
- 34.Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K. J Biol Chem. 1994;269:13685–13688. [PubMed] [Google Scholar]
- 35.Dabrowski K, Lackner R, Doblander C. Can J Fish Aquat Sci. 1990;47:1518–1525. [Google Scholar]
- 36.Burns J J, Mosbach E H, Schulenberg S. J Biol Chem. 1954;207:679–687. [PubMed] [Google Scholar]
- 37.Curtin C O, King C G. J Biol Chem. 1955;216:539–548. [PubMed] [Google Scholar]
- 38.Nelson JS. Fishes of the World. 3rd Ed. New York: Wiley; 1994. [Google Scholar]