Abstract
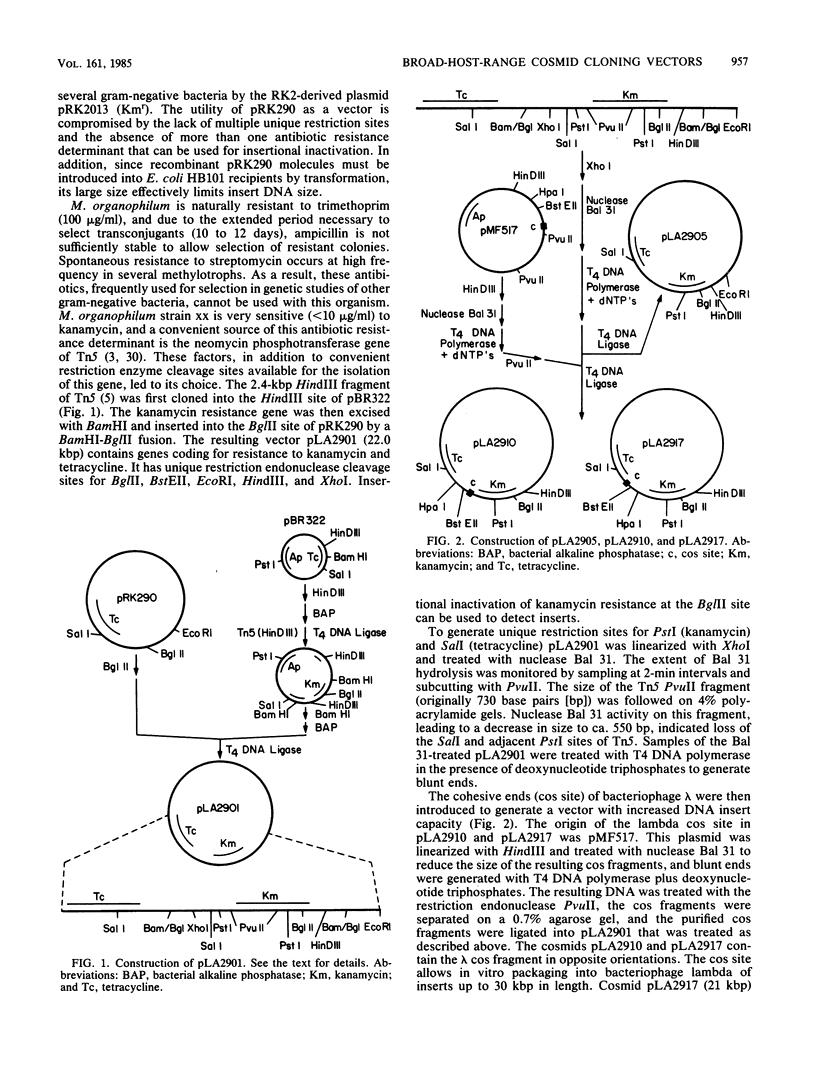
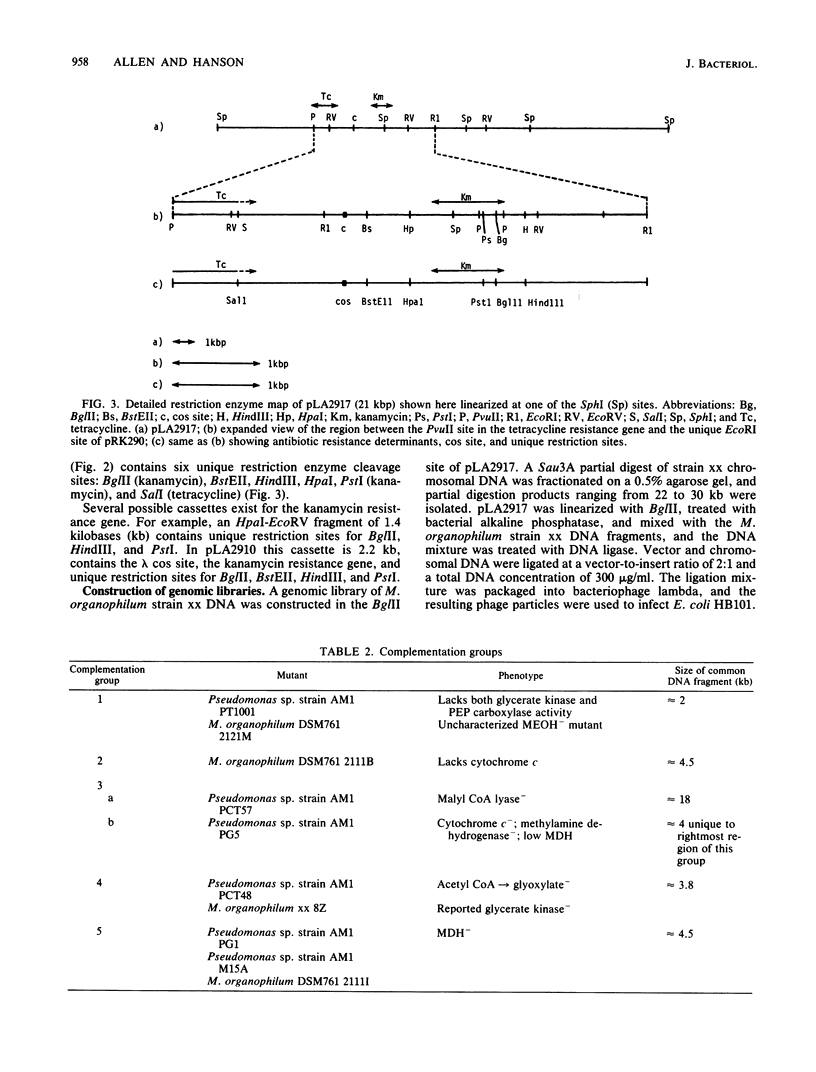
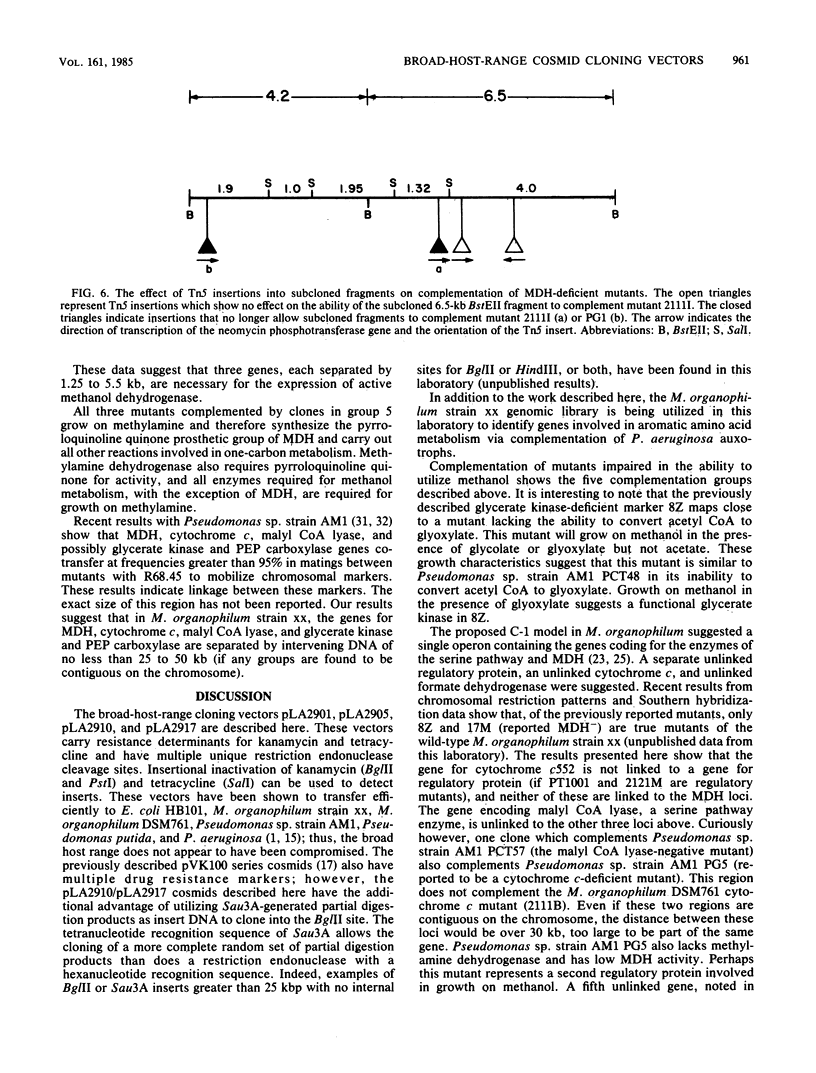
Four new cloning vectors have been constructed from the broad-host-range cloning vector pRK290. These vectors, pLA2901, pLA2905, pLA2910, and pLA2917, confer resistance to kanamycin and tetracycline. The latter two are cosmid derivatives of pLA2901. The new vectors can be mobilized into, and are stably maintained in, a variety of gram-negative bacteria. A Sau3A genomic bank of Methylobacterium organophilum strain xx DNA has been constructed in pLA2917, and complementation analysis, with a variety of mutants unable to grow on methanol, revealed at least five separate regions necessary for growth on methanol. Complementation analysis and Tn5 mutagenesis data suggest that at least three genes are responsible for expression of active methanol dehydrogenase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Bellion E., Spain J. C. The distribution of the isocitrate lyase serine pathway amongst one-carbon utilizing organisms. Can J Microbiol. 1976 Mar;22(3):404–408. doi: 10.1139/m76-061. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Daniels D. L., de Wet J. R., Blattner F. R. New map of bacteriophage lambda DNA. J Virol. 1980 Jan;33(1):390–400. doi: 10.1128/jvi.33.1.390-400.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan P. M., Anthony C., Drabble W. T. Microbial metabolism of C 1 and C 2 compounds. The role of glyoxylate, glycollate and acetate in the growth of Pseudomonas AM1 on ethanol and on C 1 compounds. Biochem J. 1972 Jun;128(1):107–115. doi: 10.1042/bj1280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C., McCann R. J. The effects of nalidixic acid on respiratory activity of asynchronous and synchronous cultures of Alcaligenes eutrophus. J Gen Microbiol. 1983 Jan;129(1):1–5. doi: 10.1099/00221287-129-1-1. [DOI] [PubMed] [Google Scholar]
- Feiss M., Siegele D. A., Rudolph C. F., Frackman S. Cosmid DNA packaging in vivo. Gene. 1982 Feb;17(2):123–130. doi: 10.1016/0378-1119(82)90064-6. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Haber C. L., Allen L. N., Zhao S., Hanson R. S. Methylotrophic bacteria: biochemical diversity and genetics. Science. 1983 Sep 16;221(4616):1147–1153. doi: 10.1126/science.221.4616.1147. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Maclennan D. G., Ousby J. C., Vasey R. B., Cotton N. T. The influence of dissolved oxygen on Pseudomonas AM1 grown on methanol in continuous culture. J Gen Microbiol. 1971 Dec;69(3):395–404. doi: 10.1099/00221287-69-3-395. [DOI] [PubMed] [Google Scholar]
- O'Connor M. L. Extension of the model concerning linkage of genes coding for C-1 related functions in Methylobacterium organophilum. Appl Environ Microbiol. 1981 Feb;41(2):437–441. doi: 10.1128/aem.41.2.437-441.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M., Wopat A., Hanson R. S. Genetic transformation in Methylobacterium organophilum. J Gen Microbiol. 1977 Jan;98(1):265–272. doi: 10.1099/00221287-98-1-265. [DOI] [PubMed] [Google Scholar]
- Ostrow R., Zachow K., Watts S., Bender M., Pass F., Faras A. Characterization of two HPV-3 related papillomaviruses from common warts that are distinct clinically from flat warts or epidermodysplasia verruciformis. J Invest Dermatol. 1983 May;80(5):436–440. doi: 10.1111/1523-1747.ep12555522. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Postle K., Reznikoff W. S. The inverted repeats of Tn5 are functionally different. Cell. 1980 Mar;19(3):795–805. doi: 10.1016/s0092-8674(80)80055-9. [DOI] [PubMed] [Google Scholar]
- Weaver C. A., Redborg A. H., Konisky J. Plasmid-determined immunity of Escherichia coli K-12 to colicin Ia Is mediated by a plasmid-encoded membrane protein. J Bacteriol. 1981 Dec;148(3):817–828. doi: 10.1128/jb.148.3.817-828.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]


