Abstract
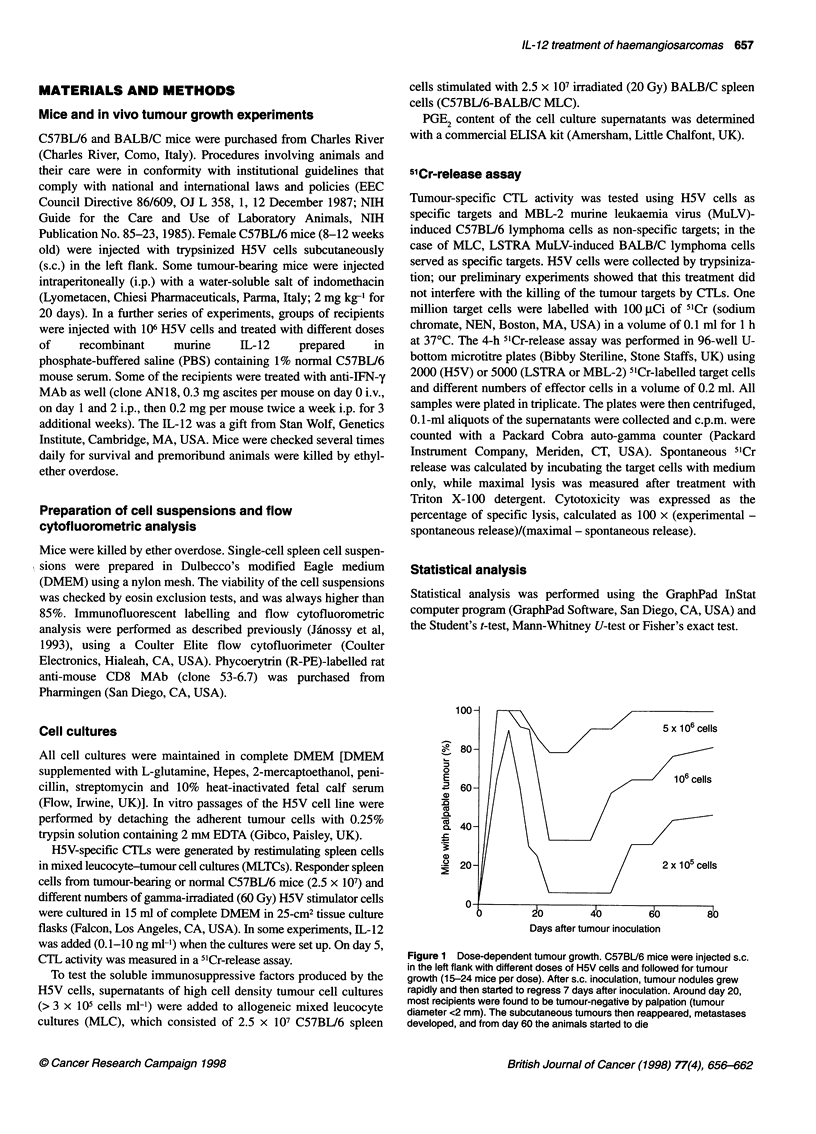
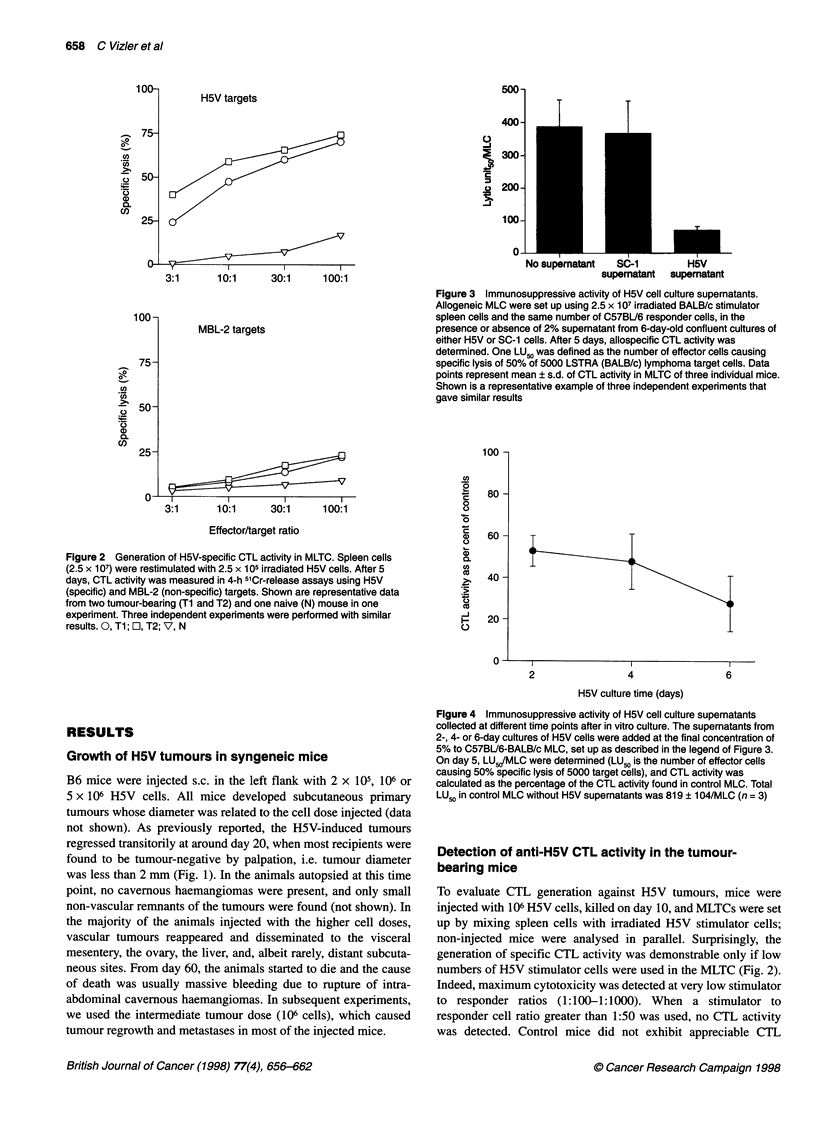
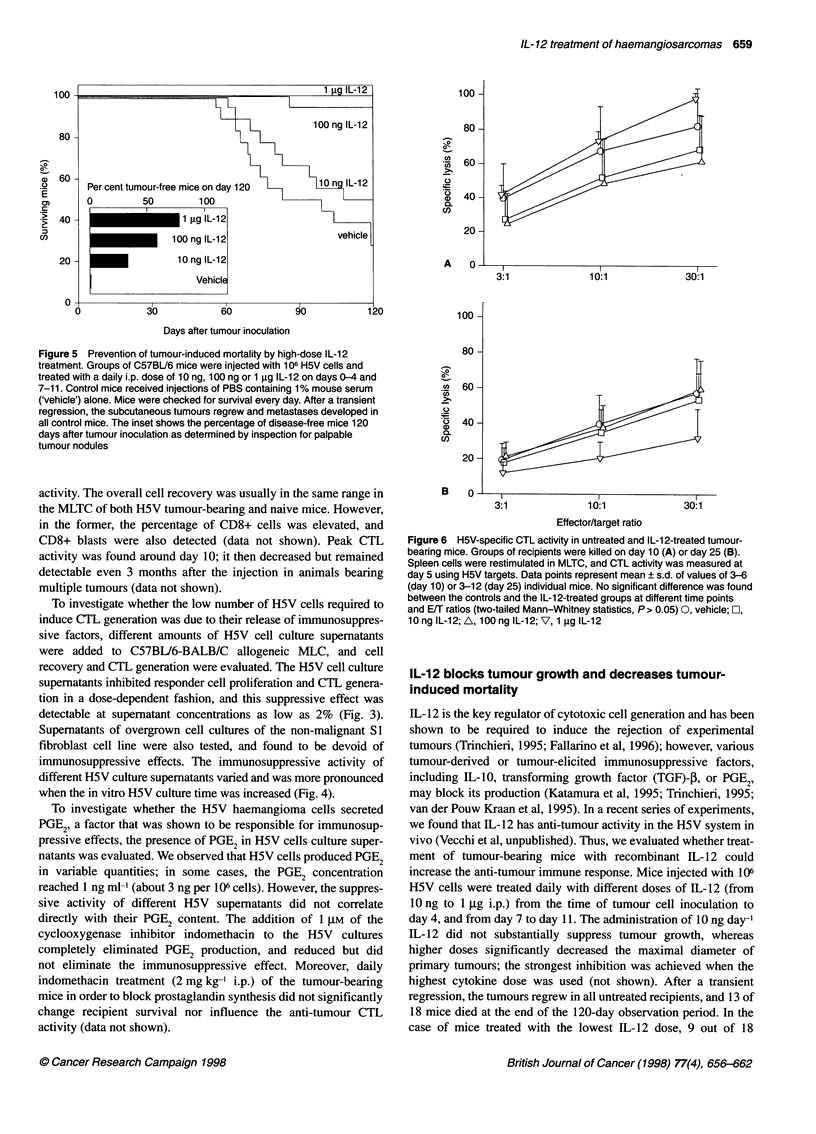
In syngeneic mice, the H5V polyoma middle-T oncogene-transformed endothelioma cell line induces Kaposi's sarcoma-like cavernous haemangiomas that regress transiently, probably because of an anti-tumour immune response, but eventually grow progressively and kill the host. To evaluate the generation of tumour-specific cytotoxic T lymphocytes (CTLs), spleen cells of tumour-bearing mice were restimulated with irradiated H5V cells in mixed leucocyte-tumour cell cultures. Tumour-specific CTLs were demonstrable only when low numbers of H5V stimulator cells were used (<1 H5V cell per 50 splenocytes). We found that H5V cells secrete immunosuppressive mediators because CTL generation was blocked when H5V cells culture supernatants were added to allogeneic mixed leucocyte cultures. As numerous tumour-derived immunosuppressive mediators may interfere with interleukin 12 (IL-12) production, we tested whether IL-12 treatment of the tumour-bearing mice would augment their immune response and thus suppress tumour growth. Indeed, IL-12 inhibited tumour growth and prevented mortality, but did not increase anti-H5V CTL generation either in vitro or in vivo. Moreover, the anti-tumour activity in IL-12-treated mice was abrogated by anti-interferon (IFN)-gamma monoclonal antibody (MAb) co-administration. These results strongly suggest that the anti-tumour effect of IL-12 is principally mediated by IFN-gamma release that in turn blocks H5V cell proliferation and induces the release of factors that suppress angiogenesis.
Full text
PDF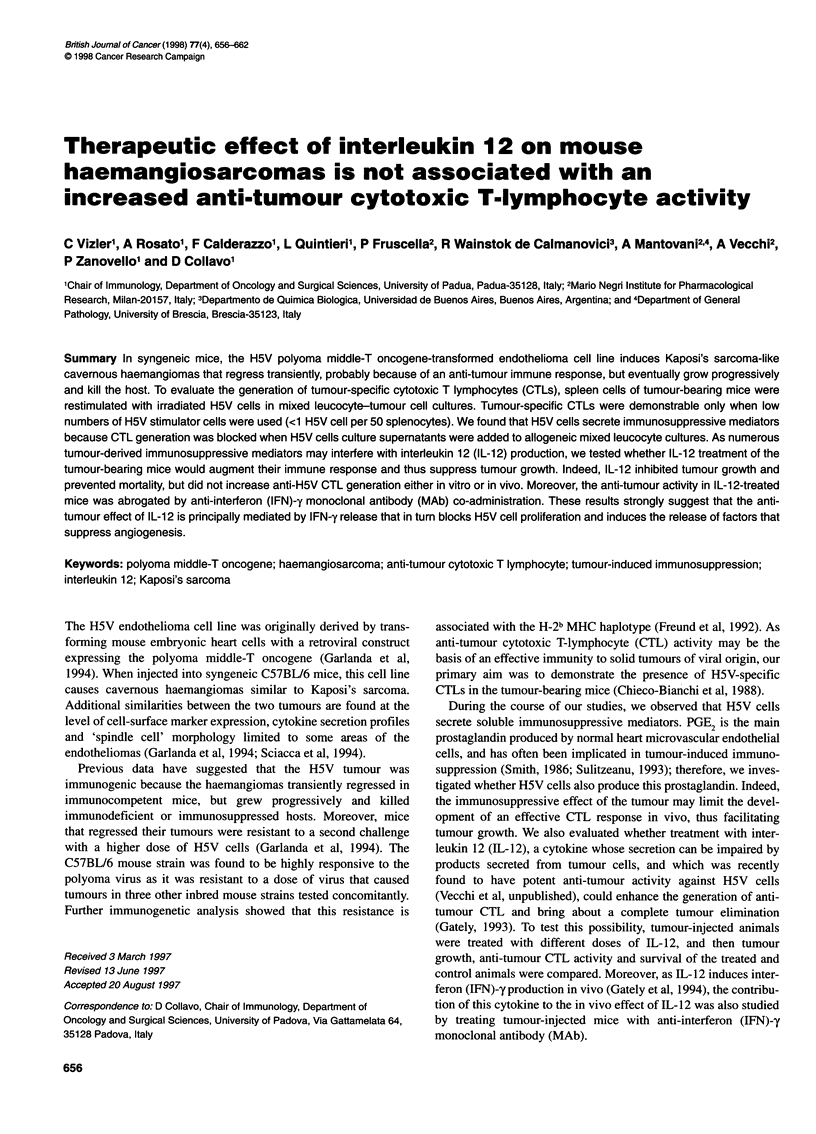


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrus J. L., Stoll H. L., Klein E. A., Karakousis C. P., Stadler S. Increased prostaglandin E2 and cAMP phosphodiesterase levels in Kaposi's sarcoma--a virus against host defense mechanism. Res Commun Chem Pathol Pharmacol. 1992 Nov;78(2):249–252. [PubMed] [Google Scholar]
- Angiolillo A. L., Sgadari C., Taub D. D., Liao F., Farber J. M., Maheshwari S., Kleinman H. K., Reaman G. H., Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995 Jul 1;182(1):155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Av P., Crofford L. J., Wilder R. L., Hla T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995 Sep 18;372(1):83–87. doi: 10.1016/0014-5793(95)00956-a. [DOI] [PubMed] [Google Scholar]
- Biron C. A., Gazzinelli R. T. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr Opin Immunol. 1995 Aug;7(4):485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- Brunda M. J., Luistro L., Warrier R. R., Wright R. B., Hubbard B. R., Murphy M., Wolf S. F., Gately M. K. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993 Oct 1;178(4):1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieco-Bianchi L., Collavo D., Biasi G. Immunologic unresponsiveness to murine leukemia virus antigens: mechanisms and role in tumor development. Adv Cancer Res. 1988;51:277–306. doi: 10.1016/s0065-230x(08)60224-9. [DOI] [PubMed] [Google Scholar]
- Dalianis T. Studies on the polyoma virus tumor-specific transplantation antigen (TSTA). Adv Cancer Res. 1990;55:57–85. doi: 10.1016/s0065-230x(08)60468-6. [DOI] [PubMed] [Google Scholar]
- Dong Q. G., Graziani A., Garlanda C., De Calmanovici R. W., Arese M., Soldi R., Vecchi A., Mantovani A., Bussolino F. Anti-tumor activity of cytokines against opportunistic vascular tumors in mice. Int J Cancer. 1996 Mar 1;65(5):700–708. doi: 10.1002/(SICI)1097-0215(19960301)65:5<700::AID-IJC23>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Fallarino F., Uyttenhove C., Boon T., Gajewski T. F. Endogenous IL-12 is necessary for rejection of P815 tumor variants in vivo. J Immunol. 1996 Feb 1;156(3):1095–1100. [PubMed] [Google Scholar]
- Freund R., Dubensky T., Bronson R., Sotnikov A., Carroll J., Benjamin T. Polyoma tumorigenesis in mice: evidence for dominant resistance and dominant susceptibility genes of the host. Virology. 1992 Dec;191(2):724–731. doi: 10.1016/0042-6822(92)90248-n. [DOI] [PubMed] [Google Scholar]
- Garlanda C., Parravicini C., Sironi M., De Rossi M., Wainstok de Calmanovici R., Carozzi F., Bussolino F., Colotta F., Mantovani A., Vecchi A. Progressive growth in immunodeficient mice and host cell recruitment by mouse endothelial cells transformed by polyoma middle-sized T antigen: implications for the pathogenesis of opportunistic vascular tumors. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7291–7295. doi: 10.1073/pnas.91.15.7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately M. K. Interleukin-12: a recently discovered cytokine with potential for enhancing cell-mediated immune responses to tumors. Cancer Invest. 1993;11(4):500–506. doi: 10.3109/07357909309018881. [DOI] [PubMed] [Google Scholar]
- Gately M. K., Warrier R. R., Honasoge S., Carvajal D. M., Faherty D. A., Connaughton S. E., Anderson T. D., Sarmiento U., Hubbard B. R., Murphy M. Administration of recombinant IL-12 to normal mice enhances cytolytic lymphocyte activity and induces production of IFN-gamma in vivo. Int Immunol. 1994 Jan;6(1):157–167. doi: 10.1093/intimm/6.1.157. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Perry L. L., Kinney-Thomas E., Benjamin T. L. Specific thymus-derived (T) cell recognition of papova virus-transformed cells. J Immunol. 1982 Feb;128(2):732–736. [PubMed] [Google Scholar]
- Hall S. S. IL-12 at the crossroads. Science. 1995 Jun 9;268(5216):1432–1434. doi: 10.1126/science.7770767. [DOI] [PubMed] [Google Scholar]
- Jánossy T., Baranyi L., Knulst A. C., Vizler C., Benner R., Kelényi G., Végh P. Autoimmunity, hyporeactivity to T cell mitogens and lymphoproliferative disorders following neonatal induction of transplantation tolerance in mice. Eur J Immunol. 1993 Nov;23(11):3011–3020. doi: 10.1002/eji.1830231143. [DOI] [PubMed] [Google Scholar]
- Katamura K., Shintaku N., Yamauchi Y., Fukui T., Ohshima Y., Mayumi M., Furusho K. Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-gamma and IL-2, but not IL-4 and IL-5. J Immunol. 1995 Nov 15;155(10):4604–4612. [PubMed] [Google Scholar]
- Liunggren G., Liunggren H. G., Dalianis T. T cell subsets involved in immunity against polyoma virus-induced tumors. Virology. 1994 Feb;198(2):714–716. doi: 10.1006/viro.1994.1084. [DOI] [PubMed] [Google Scholar]
- Luster A. D., Leder P. IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med. 1993 Sep 1;178(3):1057–1065. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Bussolino F., Introna M. Cytokine regulation of endothelial cell function: from molecular level to the bedside. Immunol Today. 1997 May;18(5):231–240. doi: 10.1016/s0167-5699(97)81662-3. [DOI] [PubMed] [Google Scholar]
- Nastala C. L., Edington H. D., McKinney T. G., Tahara H., Nalesnik M. A., Brunda M. J., Gately M. K., Wolf S. F., Schreiber R. D., Storkus W. J. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994 Aug 15;153(4):1697–1706. [PubMed] [Google Scholar]
- Noguchi Y., Richards E. C., Chen Y. T., Old L. J. Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2219–2223. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholdsson-Ljunggren G., Ramqvist T., Ahrlund-Richter L., Dalianis T. Immunization against polyoma tumors with synthetic peptides derived from the sequences of middle- and large-T antigens. Int J Cancer. 1992 Jan 2;50(1):142–146. doi: 10.1002/ijc.2910500128. [DOI] [PubMed] [Google Scholar]
- Sciacca F. L., Stürzl M., Bussolino F., Sironi M., Brandstetter H., Zietz C., Zhou D., Matteucci C., Peri G., Sozzani S. Expression of adhesion molecules, platelet-activating factor, and chemokines by Kaposi's sarcoma cells. J Immunol. 1994 Nov 15;153(10):4816–4825. [PubMed] [Google Scholar]
- Smith W. L. Prostaglandin biosynthesis and its compartmentation in vascular smooth muscle and endothelial cells. Annu Rev Physiol. 1986;48:251–262. doi: 10.1146/annurev.ph.48.030186.001343. [DOI] [PubMed] [Google Scholar]
- Sulitzeanu D. Immunosuppressive factors in human cancer. Adv Cancer Res. 1993;60:247–267. doi: 10.1016/s0065-230x(08)60827-1. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C. S., Wicker N., Armstrong D., Tubbs R., Finke J., Bukowski R. M., Hamilton T. A. Cytokine and chemokine expression in tumors of mice receiving systemic therapy with IL-12. J Immunol. 1996 Jan 15;156(2):693–699. [PubMed] [Google Scholar]
- Teicher B. A., Ara G., Menon K., Schaub R. G. In vivo studies with interleukin-12 alone and in combination with monocyte colony-stimulating factor and/or fractionated radiation treatment. Int J Cancer. 1996 Jan 3;65(1):80–84. doi: 10.1002/(SICI)1097-0215(19960103)65:1<80::AID-IJC14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- Voest E. E., Kenyon B. M., O'Reilly M. S., Truitt G., D'Amato R. J., Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995 Apr 19;87(8):581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- van der Pouw Kraan T. C., Boeije L. C., Smeenk R. J., Wijdenes J., Aarden L. A. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995 Feb 1;181(2):775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]


