Abstract
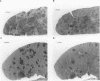
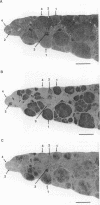
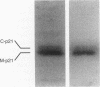
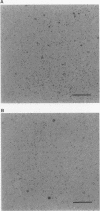
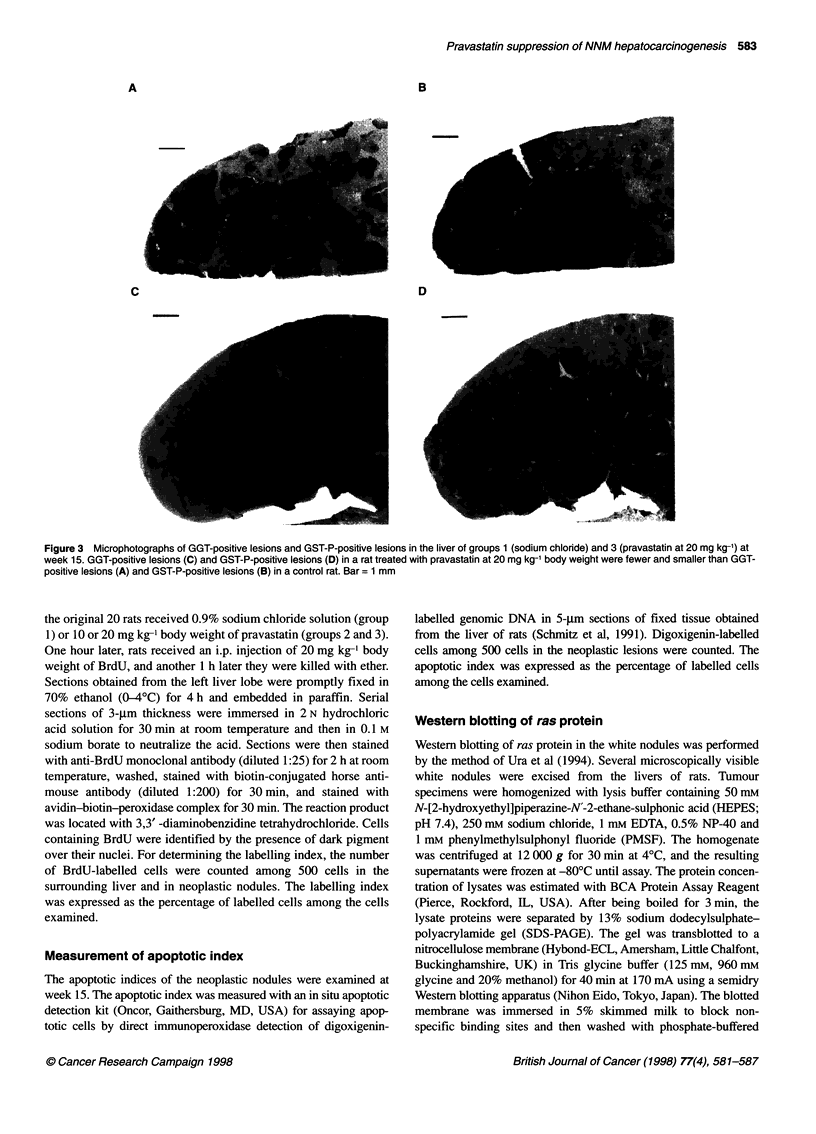
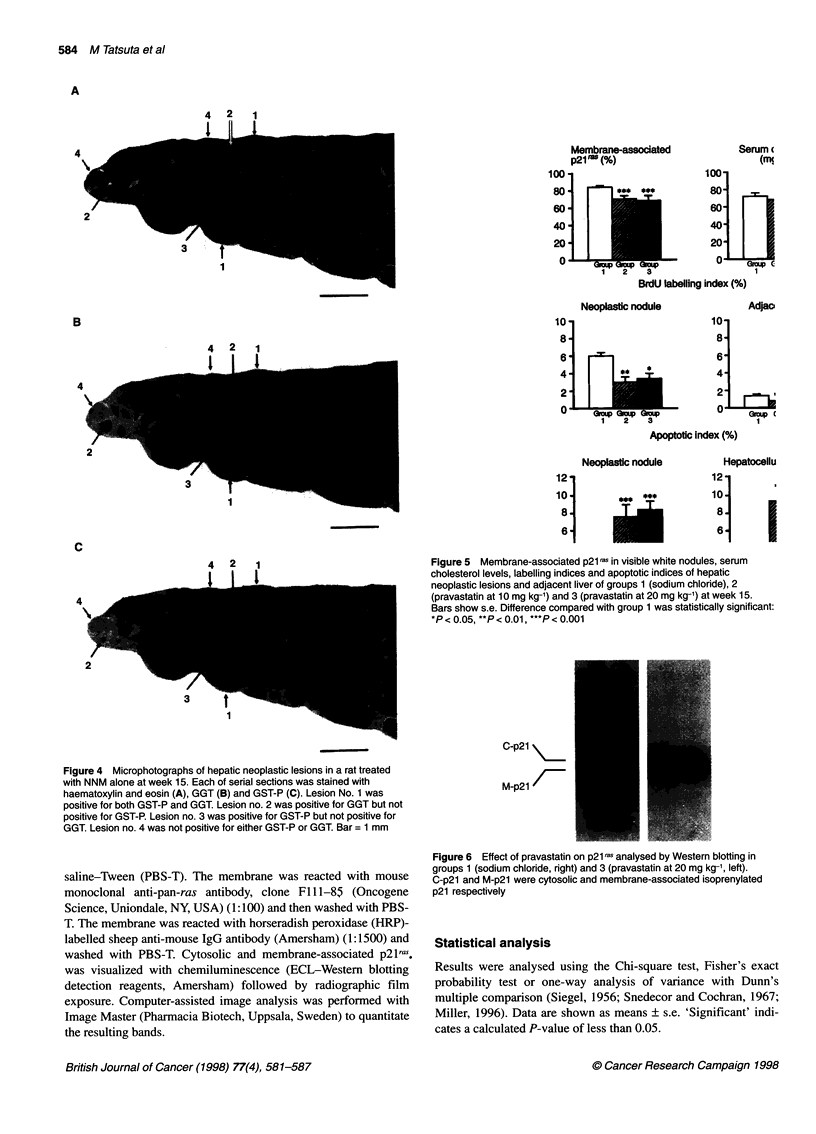
The effect of pravastatin, an inhibitor of p21ras isoprenylation, on hepatocarcinogenesis induced by N-nitrosomorpholine and on p21ras isoprenylation were investigated in male Sprague-Dawley rats. Rats received i.p. injections of pravastatin (10 and 20 mg kg(-1) body weight) every other day and, from the beginning of the experiment, were given drinking water containing N-nitrosomorpholine for 8 weeks. Visible white nodules and hepatic lesions staining positively for gamma-glutamyl transpeptidase or glutathione-S-transferase, placental type, were examined macroscopically or histochemically. In week 15, pravastatin at both dosages significantly reduced the incidence, number and volume of visible white nodules. Quantitative histological analysis also showed that prolonged administration of pravastatin at both dosages resulted in significant reductions in the number and percentage area of hepatic lesions positive for gamma-glutamyl transpeptidase and glutathione-S-transferase, placental type. Administration of pravastatin also significantly decreased the amount of membrane-associated p21ras in the tumour and the labelling index of neoplastic nodules and increased the apoptoic indices of neoplastic nodules. These findings indicate that pravastatin suppresses hepatocarcinogenesis and suggest that this effect might be related to pravastatin's inhibition of p21ras isoprenylation and its subsequent inhibition of cell proliferation and induction of apoptosis in neoplastic lesions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba M., Yamamoto R., Iishi H., Tatsuta M. Ha-ras mutations in N-nitrosomorpholine-induced lesions and inhibition of hepatocarcinogenesis by antisense sequences in rat liver. Int J Cancer. 1997 Sep 4;72(5):815–820. doi: 10.1002/(sici)1097-0215(19970904)72:5<815::aid-ijc18>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Buchwald H. Cholesterol inhibition, cancer, and chemotherapy. Lancet. 1992 May 9;339(8802):1154–1156. doi: 10.1016/0140-6736(92)90744-n. [DOI] [PubMed] [Google Scholar]
- Cox A. D., Der C. J. Protein prenylation: more than just glue? Curr Opin Cell Biol. 1992 Dec;4(6):1008–1016. doi: 10.1016/0955-0674(92)90133-w. [DOI] [PubMed] [Google Scholar]
- Dalton M. B., Fantle K. S., Bechtold H. A., DeMaio L., Evans R. M., Krystosek A., Sinensky M. The farnesyl protein transferase inhibitor BZA-5B blocks farnesylation of nuclear lamins and p21ras but does not affect their function or localization. Cancer Res. 1995 Aug 1;55(15):3295–3304. [PubMed] [Google Scholar]
- Egan S. E., Weinberg R. A. The pathway to signal achievement. Nature. 1993 Oct 28;365(6449):781–783. doi: 10.1038/365781a0. [DOI] [PubMed] [Google Scholar]
- Fairbanks K. P., Witte L. D., Goodman D. S. Relationship between mevalonate and mitogenesis in human fibroblasts stimulated with platelet-derived growth factor. J Biol Chem. 1984 Feb 10;259(3):1546–1551. [PubMed] [Google Scholar]
- Goldstein J. L., Helgeson J. A., Brown M. S. Inhibition of cholesterol synthesis with compactin renders growth of cultured cells dependent on the low density lipoprotein receptor. J Biol Chem. 1979 Jun 25;254(12):5403–5409. [PubMed] [Google Scholar]
- Grand R. J., Owen D. The biochemistry of ras p21. Biochem J. 1991 Nov 1;279(Pt 3):609–631. doi: 10.1042/bj2790609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Gu J. R., Hu L. F., Cheng Y. C., Wan D. F. Oncogenes in human primary hepatic cancer. J Cell Physiol Suppl. 1986;4:13–20. [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., Ross R. Relation of cholesterol and mevalonic acid to the cell cycle in smooth muscle and swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1980 Jun 10;255(11):5134–5140. [PubMed] [Google Scholar]
- Herold G., Jungwirth R., Rogler G., Geerling I., Stange E. F. Influence of cholesterol supply on cell growth and differentiation in cultured enterocytes (CaCo-2). Digestion. 1995;56(1):57–66. doi: 10.1159/000201223. [DOI] [PubMed] [Google Scholar]
- Kawata S., Kakimoto H., Ishiguro H., Yamasaki E., Inui Y., Matsuzawa Y. Effect of pravastatin, a potent 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, on survival of AH130 hepatoma-bearing rats. Jpn J Cancer Res. 1992 Nov;83(11):1120–1123. doi: 10.1111/j.1349-7006.1992.tb02732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata S., Nagase T., Yamasaki E., Ishiguro H., Matsuzawa Y. Modulation of the mevalonate pathway and cell growth by pravastatin and d-limonene in a human hepatoma cell line (Hep G2). Br J Cancer. 1994 Jun;69(6):1015–1020. doi: 10.1038/bjc.1994.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Willumsen B. M. Function and regulation of ras. Annu Rev Biochem. 1993;62:851–891. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- Maltese W. A., Defendini R., Green R. A., Sheridan K. M., Donley D. K. Suppression of murine neuroblastoma growth in vivo by mevinolin, a competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Clin Invest. 1985 Nov;76(5):1748–1754. doi: 10.1172/JCI112165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A. Induction of differentiation in murine neuroblastoma cells by mevinolin, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochem Biophys Res Commun. 1984 Apr 30;120(2):454–460. doi: 10.1016/0006-291x(84)91275-0. [DOI] [PubMed] [Google Scholar]
- McCormick F. Signal transduction. How receptors turn Ras on. Nature. 1993 May 6;363(6424):15–16. doi: 10.1038/363015a0. [DOI] [PubMed] [Google Scholar]
- Morris T. J., Palm S. L., Furcht L. L., Buchwald H. Effect of lovastatin alone and as an adjuvant chemotherapeutic agent on hepatoma tissue culture-4 cell growth. Ann Surg Oncol. 1995 May;2(3):266–274. doi: 10.1007/BF02307034. [DOI] [PubMed] [Google Scholar]
- Morstyn G., Hsu S. M., Kinsella T., Gratzner H., Russo A., Mitchell J. B. Bromodeoxyuridine in tumors and chromosomes detected with a monoclonal antibody. J Clin Invest. 1983 Nov;72(5):1844–1850. doi: 10.1172/JCI111145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa T., Fukaura Y., Terada K., Umezawa A., Tanida N., Yazawa K., Ishikawa C. Prevention of 1,2-dimethylhydrazine-induced colon tumorigenesis by HMG-CoA reductase inhibitors, pravastatin and simvastatin, in ICR mice. Carcinogenesis. 1994 Sep;15(9):2045–2048. doi: 10.1093/carcin/15.9.2045. [DOI] [PubMed] [Google Scholar]
- Robison R. L., Suter W., Cox R. H. Carcinogenicity and mutagenicity studies with fluvastatin, a new, entirely synthetic HMG-CoA reductase inhibitor. Fundam Appl Toxicol. 1994 Jul;23(1):9–20. doi: 10.1006/faat.1994.1073. [DOI] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Schmitz G. G., Walter T., Seibl R., Kessler C. Nonradioactive labeling of oligonucleotides in vitro with the hapten digoxigenin by tailing with terminal transferase. Anal Biochem. 1991 Jan;192(1):222–231. doi: 10.1016/0003-2697(91)90212-c. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Sumi S., Beauchamp R. D., Townsend C. M., Jr, Pour P. M., Ishizuka J., Thompson J. C. Lovastatin inhibits pancreatic cancer growth regardless of RAS mutation. Pancreas. 1994 Sep;9(5):657–661. doi: 10.1097/00006676-199409000-00018. [DOI] [PubMed] [Google Scholar]
- Sumi S., Beauchamp R. D., Townsend C. M., Jr, Uchida T., Murakami M., Rajaraman S., Ishizuka J., Thompson J. C. Inhibition of pancreatic adenocarcinoma cell growth by lovastatin. Gastroenterology. 1992 Sep;103(3):982–989. doi: 10.1016/0016-5085(92)90032-t. [DOI] [PubMed] [Google Scholar]
- Ura H., Obara T., Nishino N., Tanno S., Okamura K., Namiki M. Cytotoxicity of simvastatin to pancreatic adenocarcinoma cells containing mutant ras gene. Jpn J Cancer Res. 1994 Jun;85(6):633–638. doi: 10.1111/j.1349-7006.1994.tb02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]