Abstract
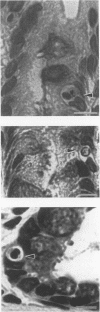
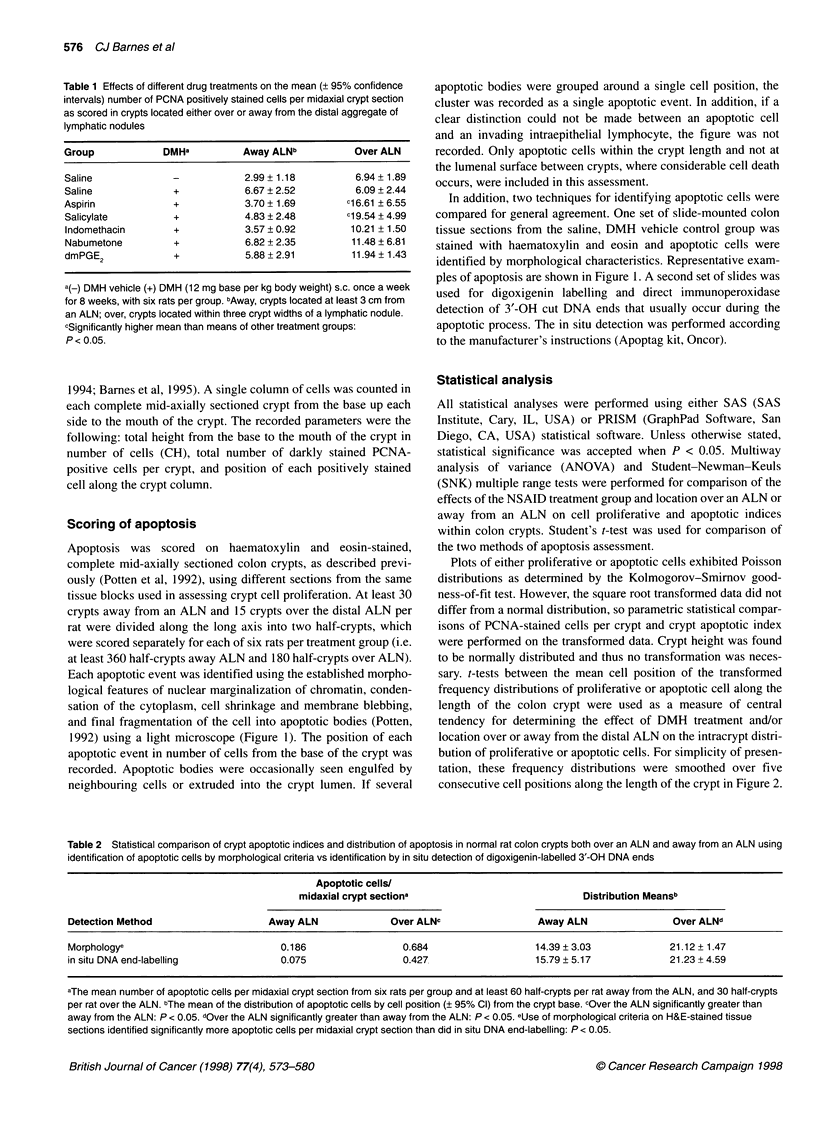
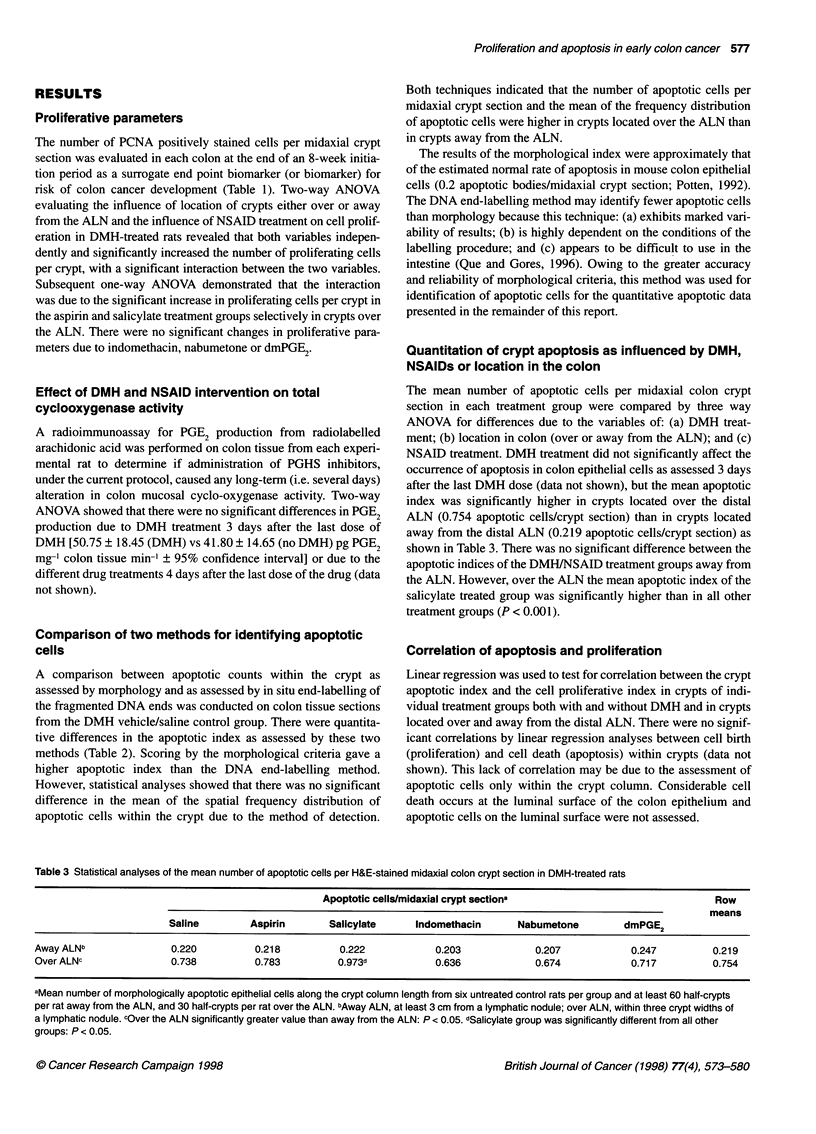
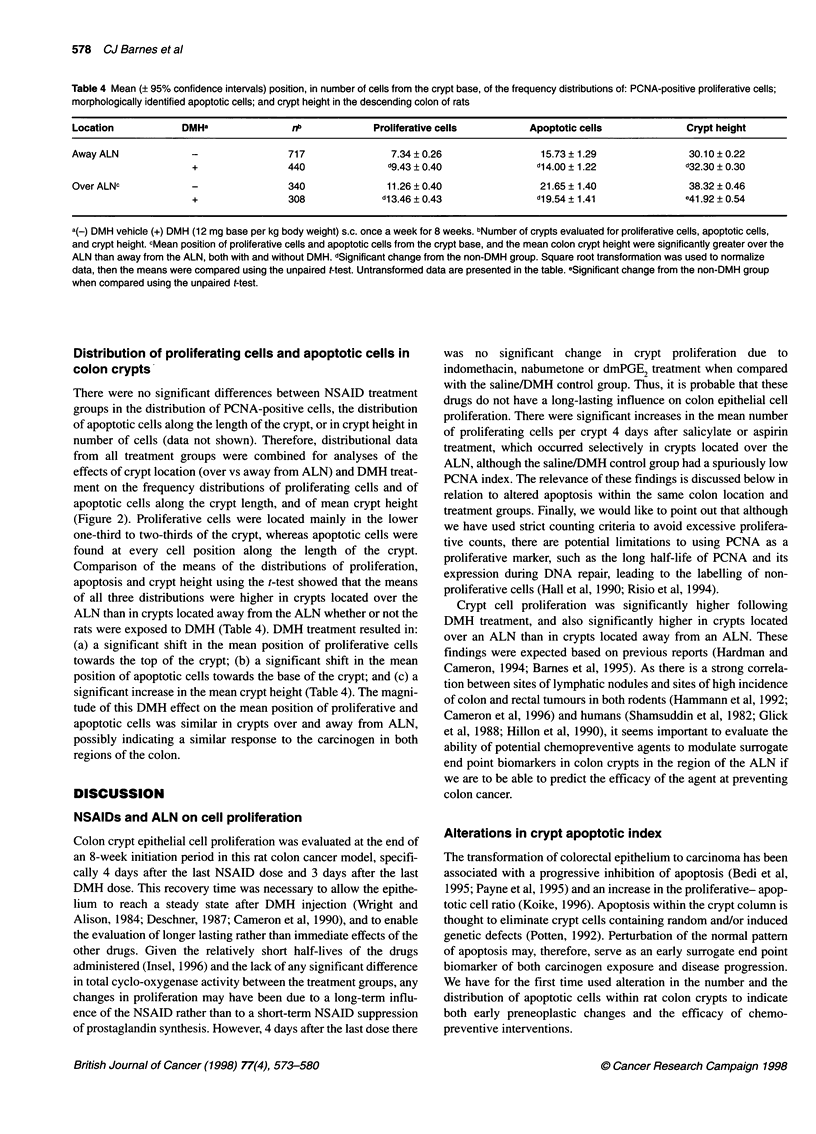
Sustained use of non-steroidal anti-inflammatory drugs (NSAIDs) may prevent colorectal cancer. However, the optimal drug, period of efficacy and mechanism(s) of action are unknown. Experiments were undertaken to determine which of several NSAIDs would modulate colon crypt cell proliferation or apoptosis when given during the initiation phase of 1,2-dimethylhydrazine (DMH)-induced rat colon cancer. Colon crypts located both away from and over an aggregate of lymphoid nodules (ALN) were examined. Rats were injected with aspirin, indomethacin, nabumetone, sodium salicylate, 16,16-dimethyl prostaglandin E2 or saline for 3 days and DMH or DMH vehicle on day 4 of each week for 8 weeks, then killed 3 days after the last DMH injection. At the time of killing, DMH had significantly increased crypt cell proliferation but not apoptosis. There was significantly more cell proliferation and apoptosis in crypts over the ALN than away from the ALN. Aspirin and salicylate increased proliferation and apoptosis in crypts over the ALN. Finally, the distributional peaks of cell proliferation and apoptosis were shifted significantly closer together after DMH. Thus, DMH increases proliferation and alters the distribution of proliferating and apoptotic cells in colon crypts early in carcinogenesis. Aspirin may suppress tumour incidence via salicylate by enhancing apoptosis in carcinogen-initiated cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes C. J., Lee M., Hardman W. E., Cameron I. L. Aspirin, age, and proximity to lymphoid nodules influence cell proliferation parameters in rat colonic crypts. Cell Prolif. 1995 Feb;28(2):59–71. doi: 10.1111/j.1365-2184.1995.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Bedi A., Pasricha P. J., Akhtar A. J., Barber J. P., Bedi G. C., Giardiello F. M., Zehnbauer B. A., Hamilton S. R., Jones R. J. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995 May 1;55(9):1811–1816. [PubMed] [Google Scholar]
- Cameron I. L., Garza J., Hardman W. E. Distribution of lymphoid nodules, aberrant crypt foci and tumours in the colon of carcinogen-treated rats. Br J Cancer. 1996 Apr;73(7):893–898. doi: 10.1038/bjc.1996.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Effects of aspirin on 1,2-dimethylhydrazine-induced colonic carcinogenesis. Carcinogenesis. 1992 Apr;13(4):541–546. doi: 10.1093/carcin/13.4.541. [DOI] [PubMed] [Google Scholar]
- Deschner E. E. Cell turnover and colon tumor development. Prev Med. 1987 Jul;16(4):580–585. doi: 10.1016/0091-7435(87)90075-2. [DOI] [PubMed] [Google Scholar]
- Deschner E. E., Maskens A. P. Significance of the labeling index and labeling distribution as kinetic parameters in colorectal mucosa of cancer patients and DMH treated animals. Cancer. 1982 Sep 15;50(6):1136–1141. doi: 10.1002/1097-0142(19820915)50:6<1136::aid-cncr2820500617>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- DuBois R. N., Radhika A., Reddy B. S., Entingh A. J. Increased cyclooxygenase-2 levels in carcinogen-induced rat colonic tumors. Gastroenterology. 1996 Apr;110(4):1259–1262. doi: 10.1053/gast.1996.v110.pm8613017. [DOI] [PubMed] [Google Scholar]
- Earnest D. L., Hixson L. J., Alberts D. S. Piroxicam and other cyclooxygenase inhibitors: potential for cancer chemoprevention. J Cell Biochem Suppl. 1992;16I:156–166. doi: 10.1002/jcb.240501330. [DOI] [PubMed] [Google Scholar]
- Eberhart C. E., Coffey R. J., Radhika A., Giardiello F. M., Ferrenbach S., DuBois R. N. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994 Oct;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Elder D. J., Hague A., Hicks D. J., Paraskeva C. Differential growth inhibition by the aspirin metabolite salicylate in human colorectal tumor cell lines: enhanced apoptosis in carcinoma and in vitro-transformed adenoma relative to adenoma relative to adenoma cell lines. Cancer Res. 1996 May 15;56(10):2273–2276. [PubMed] [Google Scholar]
- Giardiello F. M., Offerhaus G. J., DuBois R. N. The role of nonsteroidal anti-inflammatory drugs in colorectal cancer prevention. Eur J Cancer. 1995 Jul-Aug;31A(7-8):1071–1076. doi: 10.1016/0959-8049(95)00137-8. [DOI] [PubMed] [Google Scholar]
- Giovannucci E., Egan K. M., Hunter D. J., Stampfer M. J., Colditz G. A., Willett W. C., Speizer F. E. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995 Sep 7;333(10):609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- Glick S. N., Teplick S. K., Ross W. M. Colonic lymphoid follicles associated with colonic neoplasms. Radiology. 1988 Sep;168(3):603–607. doi: 10.1148/radiology.168.3.3406391. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S. Prostaglandins and host defense in cancer. Med Clin North Am. 1981 Jul;65(4):829–844. doi: 10.1016/s0025-7125(16)31500-0. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Levison D. A., Woods A. L., Yu C. C., Kellock D. B., Watkins J. A., Barnes D. M., Gillett C. E., Camplejohn R., Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990 Dec;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Hammann A., Arveux P., Martin M. Effect of gut-associated lymphoid tissue on cellular proliferation in proximal and distal colon of the rat. Dig Dis Sci. 1992 Jul;37(7):1099–1104. doi: 10.1007/BF01300293. [DOI] [PubMed] [Google Scholar]
- Hardman W. E., Cameron I. L. Colonic crypts located over lymphoid nodules of 1,2-dimethylhydrazine-treated rats are hyperplastic and at high risk of forming adenocarcinomas. Carcinogenesis. 1994 Oct;15(10):2353–2361. doi: 10.1093/carcin/15.10.2353. [DOI] [PubMed] [Google Scholar]
- Hillon P., Martin M. S., Piard F., Jacquot J. F. Relation between adenomas and colorectal-associated lymphoid tissue in familial polyposis coli. Dig Dis Sci. 1990 Oct;35(10):1307–1308. doi: 10.1007/BF01536426. [DOI] [PubMed] [Google Scholar]
- Kargman S. L., O'Neill G. P., Vickers P. J., Evans J. F., Mancini J. A., Jothy S. Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res. 1995 Jun 15;55(12):2556–2559. [PubMed] [Google Scholar]
- Koike M. Significance of spontaneous apoptosis during colorectal tumorigenesis. J Surg Oncol. 1996 Jun;62(2):97–108. doi: 10.1002/(SICI)1096-9098(199606)62:2<97::AID-JSO5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Laneuville O., Breuer D. K., Dewitt D. L., Hla T., Funk C. D., Smith W. L. Differential inhibition of human prostaglandin endoperoxide H synthases-1 and -2 by nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. 1994 Nov;271(2):927–934. [PubMed] [Google Scholar]
- Lee M., Feldman M. Nonessential role of leukotrienes as mediators of acute gastric mucosal injury induced by aspirin in rats. Dig Dis Sci. 1992 Aug;37(8):1282–1287. doi: 10.1007/BF01296573. [DOI] [PubMed] [Google Scholar]
- Marnett L. J. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res. 1992 Oct 15;52(20):5575–5589. [PubMed] [Google Scholar]
- Meade E. A., Smith W. L., DeWitt D. L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993 Mar 25;268(9):6610–6614. [PubMed] [Google Scholar]
- Merritt A. J., Potten C. S., Watson A. J., Loh D. Y., Nakayama K., Nakayama K., Hickman J. A. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 1995 Jun;108(Pt 6):2261–2271. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- Pasricha P. J., Bedi A., O'Connor K., Rashid A., Akhtar A. J., Zahurak M. L., Piantadosi S., Hamilton S. R., Giardiello F. M. The effects of sulindac on colorectal proliferation and apoptosis in familial adenomatous polyposis. Gastroenterology. 1995 Sep;109(3):994–998. doi: 10.1016/0016-5085(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Payne C. M., Bernstein H., Bernstein C., Garewal H. Role of apoptosis in biology and pathology: resistance to apoptosis in colon carcinogenesis. Ultrastruct Pathol. 1995 Jul-Aug;19(4):221–248. doi: 10.3109/01913129509064227. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Li Y. Q., O'Connor P. J., Winton D. J. A possible explanation for the differential cancer incidence in the intestine, based on distribution of the cytotoxic effects of carcinogens in the murine large bowel. Carcinogenesis. 1992 Dec;13(12):2305–2312. doi: 10.1093/carcin/13.12.2305. [DOI] [PubMed] [Google Scholar]
- Potten C. S. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev. 1992 Sep;11(2):179–195. doi: 10.1007/BF00048063. [DOI] [PubMed] [Google Scholar]
- Pugh S., Thomas G. A. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E2. Gut. 1994 May;35(5):675–678. doi: 10.1136/gut.35.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L., Kozoni V., Tsioulias G. J., Koutsos M. I., Hanif R., Shiff S. J., Rigas B. Selected eicosanoids increase the proliferation rate of human colon carcinoma cell lines and mouse colonocytes in vivo. Biochim Biophys Acta. 1995 Sep 14;1258(2):215–223. doi: 10.1016/0005-2760(95)00100-q. [DOI] [PubMed] [Google Scholar]
- Que F. G., Gores G. J. Cell death by apoptosis: basic concepts and disease relevance for the gastroenterologist. Gastroenterology. 1996 Apr;110(4):1238–1243. doi: 10.1053/gast.1996.v110.pm8613014. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Rao C. V., Rivenson A., Kelloff G. Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F344 rats. Carcinogenesis. 1993 Aug;14(8):1493–1497. doi: 10.1093/carcin/14.8.1493. [DOI] [PubMed] [Google Scholar]
- Redfern J. S., Lee E., Feldman M. Effect of indomethacin on gastric mucosal prostaglandins in humans. Correlation with mucosal damage. Gastroenterology. 1987 Apr;92(4):969–977. doi: 10.1016/0016-5085(87)90972-3. [DOI] [PubMed] [Google Scholar]
- Risio M., Candelaresi G., Rossini F. P. Bromodeoxyuridine uptake and proliferating cell nuclear antigen expression throughout the colorectal tumor sequence. Cancer Epidemiol Biomarkers Prev. 1993 Jul-Aug;2(4):363–367. [PubMed] [Google Scholar]
- Risio M. Methodological aspects of using immunohistochemical cell proliferation biomarkers in colorectal carcinoma chemoprevention. J Cell Biochem Suppl. 1994;19:61–67. [PubMed] [Google Scholar]
- Shamsuddin A. M., Phelps P. C., Trump B. F. Human large intestinal epithelium: light microscopy, histochemistry, and ultrastructure. Hum Pathol. 1982 Sep;13(9):790–803. doi: 10.1016/s0046-8177(82)80075-0. [DOI] [PubMed] [Google Scholar]
- Shiff S. J., Qiao L., Tsai L. L., Rigas B. Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells. J Clin Invest. 1995 Jul;96(1):491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L., Dewitt D. L. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- Thun M. J. Aspirin, NSAIDs, and digestive tract cancers. Cancer Metastasis Rev. 1994 Dec;13(3-4):269–277. doi: 10.1007/BF00666097. [DOI] [PubMed] [Google Scholar]