Abstract
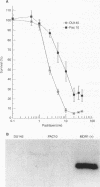
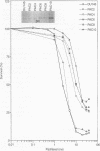
To investigate the role of beta-tubulin isotype composition in resistance to paclitaxel, an anti-microtubule agent, human prostate carcinoma (DU-145) cells were intermittently exposed to increasing concentrations of paclitaxel. Cells that were selected and maintained at 10 nM paclitaxel (Pac-10) were fivefold resistant to the drug. Pac-10 cells accumulated radiolabelled paclitaxel to the same extent as DU-145 cells and were negative for MDR-1. Analysis of Pac-10 and DU-145 cells by flow cytometry showed similar cell cycle patterns. Immunofluorescent staining revealed an overall increase of alpha- and beta-tubulin levels in Pac-10 cells compared with DU-145 cells. Examination of beta-tubulin isotype composition revealed a significant increase in betaIII isotype in the resistant cells, both by immunofluorescence and by western blot analysis. Reverse transcription-polymerase chain reaction (RT-PCR) analysis of the isotypes confirmed the increase observed for the betaIII by exhibiting ninefold higher betaIII mRNA levels and also showed fivefold increase of the betaIVa transcript. In addition, analysis of paclitaxel-resistant cells that were selected at increasing levels of the drug (Pac 2, 4, 6, 8 and 10) exhibited a positive correlation between increasing betaIII levels and increasing resistance to paclitaxel. Increased expression of specific beta-tubulin isotypes and subsequent incorporation into microtubules may alter cellular microtubule dynamics, providing a defence against the anti-microtubule effects of paclitaxel and other tubulin-binding drugs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A., Roach M. C., Trcka P., Ludueña R. F. Increased microtubule assembly in bovine brain tubulin lacking the type III isotype of beta-tubulin. J Biol Chem. 1990 Jan 25;265(3):1794–1799. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cross D., Farías G., Domínguez J., Avila J., Maccioni R. B. Carboxyl terminal sequences of beta-tubulin involved in the interaction of HMW-MAPs. Studies using site-specific antibodies. Mol Cell Biochem. 1994 Mar 16;132(1):81–90. doi: 10.1007/BF00925677. [DOI] [PubMed] [Google Scholar]
- Dahllöf B., Billström A., Cabral F., Hartley-Asp B. Estramustine depolymerizes microtubules by binding to tubulin. Cancer Res. 1993 Oct 1;53(19):4573–4581. [PubMed] [Google Scholar]
- Derry W. B., Wilson L., Jordan M. A. Substoichiometric binding of taxol suppresses microtubule dynamics. Biochemistry. 1995 Feb 21;34(7):2203–2211. doi: 10.1021/bi00007a014. [DOI] [PubMed] [Google Scholar]
- Derry W. B., Wilson L., Khan I. A., Luduena R. F., Jordan M. A. Taxol differentially modulates the dynamics of microtubules assembled from unfractionated and purified beta-tubulin isotypes. Biochemistry. 1997 Mar 25;36(12):3554–3562. doi: 10.1021/bi962724m. [DOI] [PubMed] [Google Scholar]
- Dhamodharan R., Jordan M. A., Thrower D., Wilson L., Wadsworth P. Vinblastine suppresses dynamics of individual microtubules in living interphase cells. Mol Biol Cell. 1995 Sep;6(9):1215–1229. doi: 10.1091/mbc.6.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontalba A., Avila J., Zabala J. C. Beta-tubulin folding is modulated by the isotype-specific carboxy-terminal domain. J Mol Biol. 1995 Mar 10;246(5):628–636. doi: 10.1016/s0022-2836(05)80112-3. [DOI] [PubMed] [Google Scholar]
- Haber M., Burkhart C. A., Regl D. L., Madafiglio J., Norris M. D., Horwitz S. B. Altered expression of M beta 2, the class II beta-tubulin isotype, in a murine J774.2 cell line with a high level of taxol resistance. J Biol Chem. 1995 Dec 29;270(52):31269–31275. doi: 10.1074/jbc.270.52.31269. [DOI] [PubMed] [Google Scholar]
- Hudes G. R., Nathan F., Khater C., Haas N., Cornfield M., Giantonio B., Greenberg R., Gomella L., Litwin S., Ross E. Phase II trial of 96-hour paclitaxel plus oral estramustine phosphate in metastatic hormone-refractory prostate cancer. J Clin Oncol. 1997 Sep;15(9):3156–3163. doi: 10.1200/JCO.1997.15.9.3156. [DOI] [PubMed] [Google Scholar]
- Hudes G. Estramustine-based chemotherapy. Semin Urol Oncol. 1997 Feb;15(1):13–19. [PubMed] [Google Scholar]
- Jordan M. A., Thrower D., Wilson L. Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles. Implications for the role of microtubule dynamics in mitosis. J Cell Sci. 1992 Jul;102(Pt 3):401–416. doi: 10.1242/jcs.102.3.401. [DOI] [PubMed] [Google Scholar]
- Jordan M. A., Toso R. J., Thrower D., Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing N., Dahllöf B., Hartley-Asp B., Ranganathan S., Tew K. D. Interaction of estramustine with tubulin isotypes. Biochemistry. 1997 Jan 28;36(4):871–878. doi: 10.1021/bi961445w. [DOI] [PubMed] [Google Scholar]
- Lu Q., Luduena R. F. Removal of beta III isotype enhances taxol induced microtubule assembly. Cell Struct Funct. 1993 Jun;18(3):173–182. doi: 10.1247/csf.18.173. [DOI] [PubMed] [Google Scholar]
- Panda D., Miller H. P., Banerjee A., Ludueña R. F., Wilson L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11358–11362. doi: 10.1073/pnas.91.24.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan S., Dexter D. W., Benetatos C. A., Chapman A. E., Tew K. D., Hudes G. R. Increase of beta(III)- and beta(IVa)-tubulin isotopes in human prostate carcinoma cells as a result of estramustine resistance. Cancer Res. 1996 Jun 1;56(11):2584–2589. [PubMed] [Google Scholar]
- Roth B. J., Yeap B. Y., Wilding G., Kasimis B., McLeod D., Loehrer P. J. Taxol in advanced, hormone-refractory carcinoma of the prostate. A phase II trial of the Eastern Cooperative Oncology Group. Cancer. 1993 Oct 15;72(8):2457–2460. doi: 10.1002/1097-0142(19931015)72:8<2457::aid-cncr2820720825>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990 Jul 4;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Speicher L. A., Barone L., Tew K. D. Combined antimicrotubule activity of estramustine and taxol in human prostatic carcinoma cell lines. Cancer Res. 1992 Aug 15;52(16):4433–4440. [PubMed] [Google Scholar]
- Speicher L. A., Laing N., Barone L. R., Robbins J. D., Seamon K. B., Tew K. D. Interaction of an estramustine photoaffinity analogue with cytoskeletal proteins in prostate carcinoma cells. Mol Pharmacol. 1994 Nov;46(5):866–872. [PubMed] [Google Scholar]
- Sullivan K. F. Structure and utilization of tubulin isotypes. Annu Rev Cell Biol. 1988;4:687–716. doi: 10.1146/annurev.cb.04.110188.003351. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Zilfou J. T., Smith C. D. Differential interactions of cytochalasins with P-glycoprotein. Oncol Res. 1995;7(9):435–443. [PubMed] [Google Scholar]