Abstract
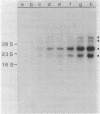
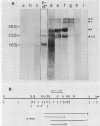
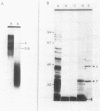
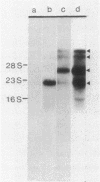
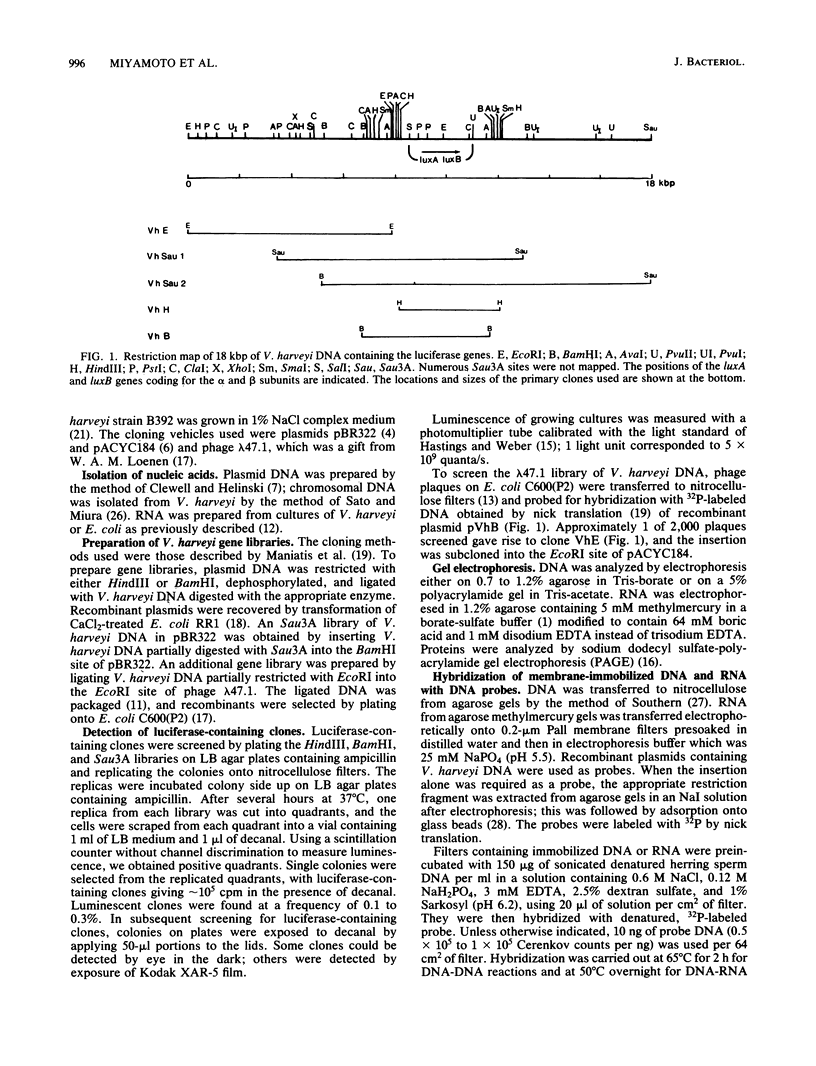
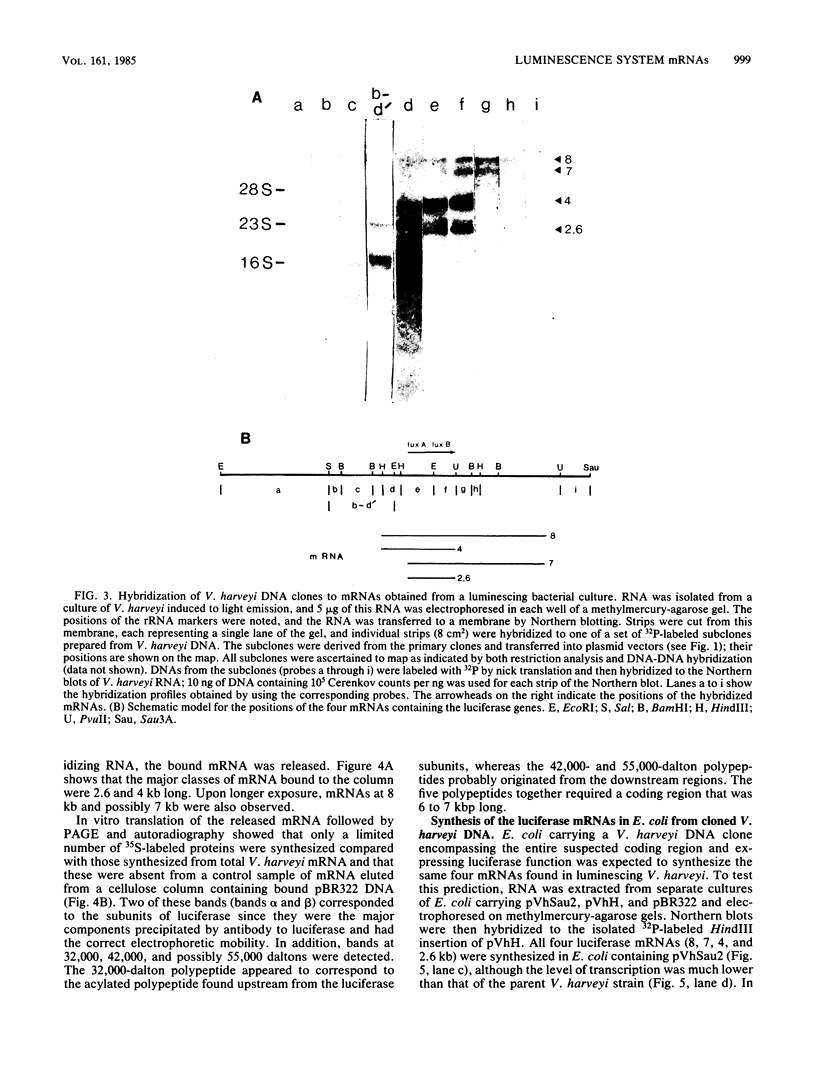
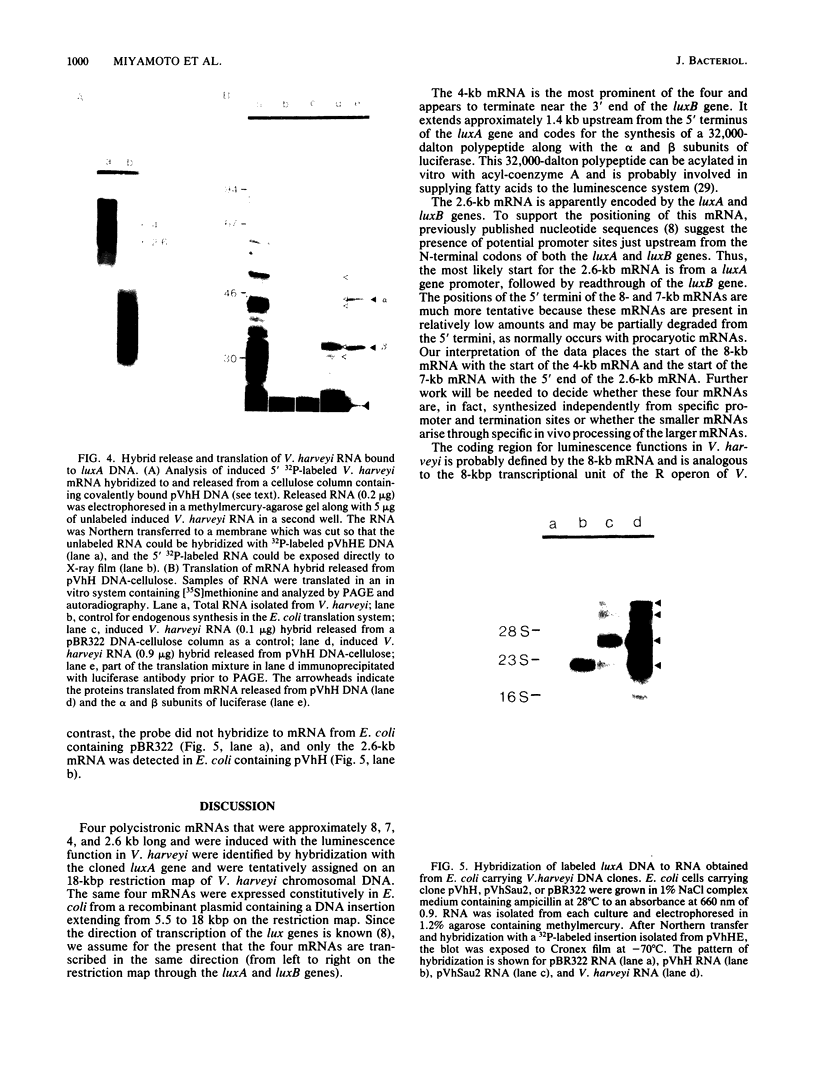
DNA coding for the alpha and beta subunits of Vibrio harveyi luciferase, the luxA and luxB genes, and the adjoining chromosomal regions on both sides of these genes (total of 18 kilobase pairs) was cloned into Escherichia coli. Using labeled DNA coding for the alpha subunit as a hybridization probe, we identified a set of polycistronic mRNAs (2.6, 4, 7, and 8 kilobases) by Northern blotting; the most prominent of these was the one 4 kilobases long. This set of mRNAs was induced during the development of bioluminescence in V. harveyi. Furthermore, the same set of mRNAs was synthesized in E. coli by a recombinant plasmid that contained a 12-kilobase pair length of V. harveyi DNA and expressed the genes for the luciferase subunits. A cloned DNA segment corresponding to the major 4-kilobase mRNA coded for the alpha and beta subunits of luciferase, as well as a 32,000-dalton protein upstream from these genes that could be specifically modified by acyl-coenzyme A and is a component of the bioluminescence system. V. harveyi mRNA that was hybridized to and released from cloned DNA encompassing the luxA and luxB genes was translated in vitro. Luciferase alpha and beta subunits and the 32,000-dalton polypeptide were detected among the products, along with 42,000- and 55,000-dalton polypeptides, which are encoded downstream from the lux genes and are thought to be involved in luminescence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Baldwin T. O., Berends T., Bunch T. A., Holzman T. F., Rausch S. K., Shamansky L., Treat M. L., Ziegler M. M. Cloning of the luciferase structural genes from Vibrio harveyi and expression of bioluminescence in Escherichia coli. Biochemistry. 1984 Jul 31;23(16):3663–3667. doi: 10.1021/bi00311a014. [DOI] [PubMed] [Google Scholar]
- Belas R., Mileham A., Cohn D., Hilman M., Simon M., Silverman M. Bacterial bioluminescence: isolation and expression of the luciferase genes from Vibrio harveyi. Science. 1982 Nov 19;218(4574):791–793. doi: 10.1126/science.10636771. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Byers D., Meighen E. Vibrio harveyi aldehyde dehydrogenase. Partial reversal of aldehyde oxidation and its possible role in the reduction of fatty acids for the bioluminescence reaction. J Biol Chem. 1984 Jun 10;259(11):7109–7114. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. H., Ogden R. C., Abelson J. N., Baldwin T. O., Nealson K. H., Simon M. I., Mileham A. J. Cloning of the Vibrio harveyi luciferase genes: use of a synthetic oligonucleotide probe. Proc Natl Acad Sci U S A. 1983 Jan;80(1):120–123. doi: 10.1073/pnas.80.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Evans J. F., McCracken S., Miyamoto C. M., Meighen E. A., Graham A. F. In vitro synthesis of subunits of bacterial luciferase in an Escherichia coli system. J Bacteriol. 1983 Jan;153(1):543–545. doi: 10.1128/jb.153.1.543-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASTINGS J. W., RILEY W. H., MASSA J. THE PURIFICATION PROPERTIES, AND CHEMILUMINESCENT QUANTUM YIELD OF BACTERIAL LUCIFERASE. J Biol Chem. 1965 Mar;240:1473–1481. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- McCracken S., Meighen E. Functional and structural properties of immobilized subunits of Escherichia coli alkaline phosphatase. J Biol Chem. 1980 Mar 25;255(6):2396–2404. [PubMed] [Google Scholar]
- Michaliszyn G. A., Meighen E. A. Induced polypeptide synthesis during the development of bacterial bioluminescence. J Biol Chem. 1976 May 10;251(9):2541–2549. [PubMed] [Google Scholar]
- Miyamoto C., Denhardt D. T. Evidence for the presence of ribonucleotides at the 5' termini of some DNA molecules isolated from Escherichia coli polAex2. J Mol Biol. 1977 Nov;116(4):681–707. doi: 10.1016/0022-2836(77)90266-2. [DOI] [PubMed] [Google Scholar]
- Moss L. G., Moore J. P., Chan L. A simple, efficient method for coupling DNA to cellulose. Development of the method and application to mRNA purification. J Biol Chem. 1981 Dec 25;256(24):12655–12658. [PubMed] [Google Scholar]
- Nealson K. H., Hastings J. W. Bacterial bioluminescence: its control and ecological significance. Microbiol Rev. 1979 Dec;43(4):496–518. doi: 10.1128/mr.43.4.496-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall L. A., Byers D. M., Meighen E. A. In vivo and in vitro acylation of polypeptides in Vibrio harveyi: identification of proteins involved in aldehyde production for bioluminescence. J Bacteriol. 1984 Aug;159(2):720–724. doi: 10.1128/jb.159.2.720-724.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall L., Rodriquez A., Meighen E. Differential acylation in vitro with tetradecanoyl coenzyme A and tetradecanoic acid (+ATP) of three polypeptides shown to have induced synthesis in Photobacterium phosphoreum. J Biol Chem. 1984 Feb 10;259(3):1409–1414. [PubMed] [Google Scholar]
- Weiss B., Live T. R., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. V. End group labeling and analysis of deoxyribonucleic acid containing single straned breaks. J Biol Chem. 1968 Sep 10;243(17):4530–4542. [PubMed] [Google Scholar]