Abstract
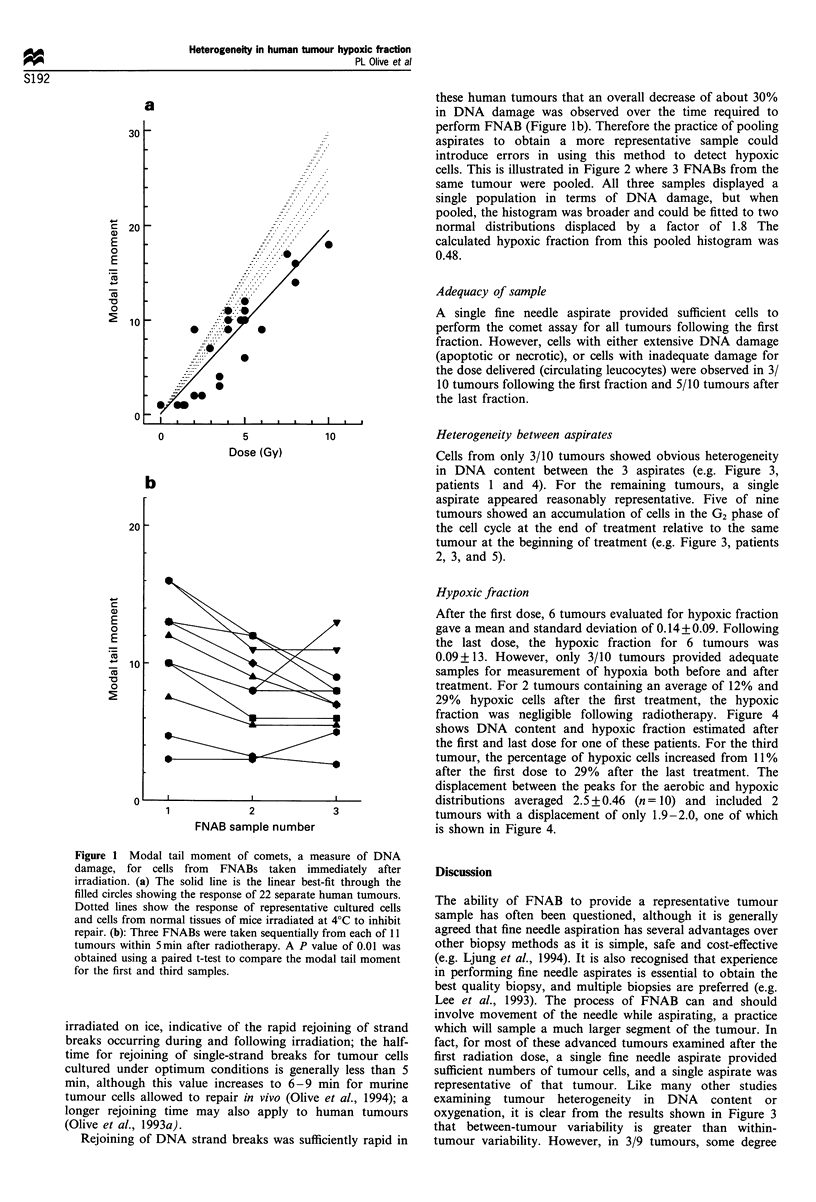
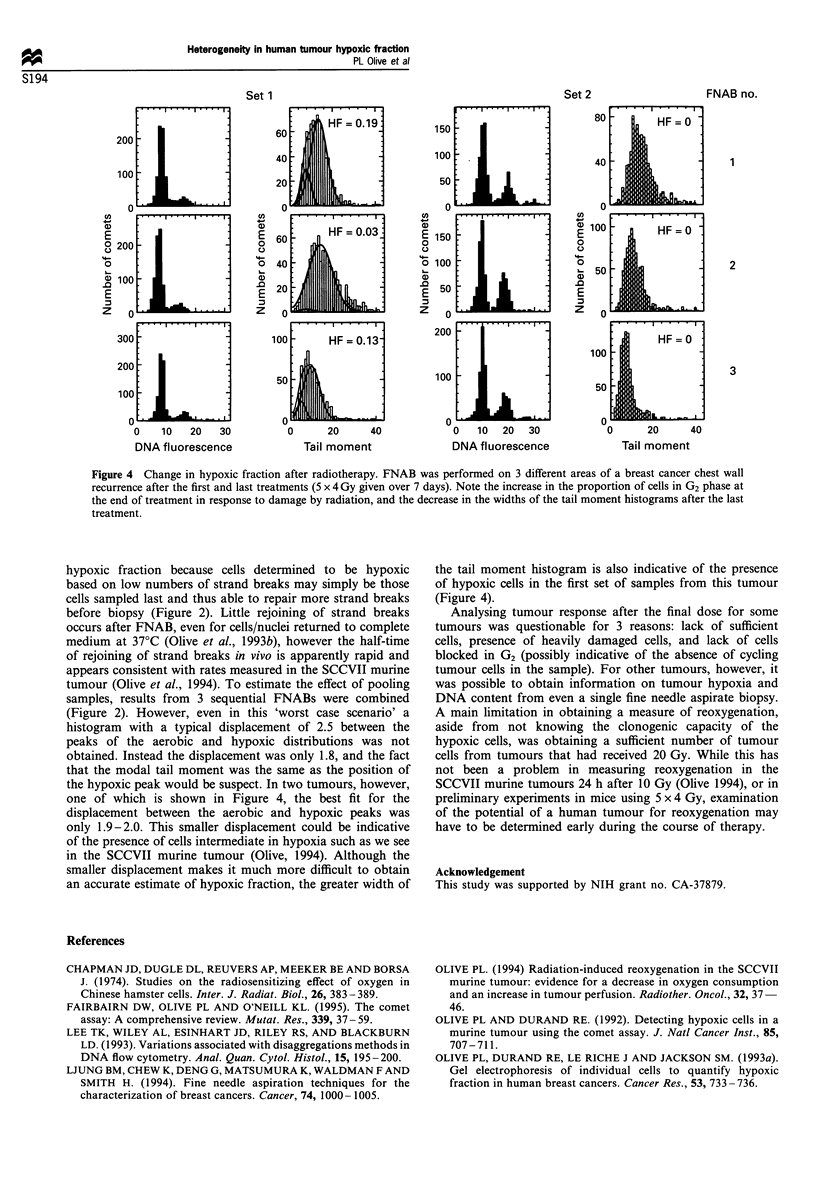
The alkaline comet assay has previously been used to estimate the fraction of radiobiologically hypoxic cells in tumours from patients undergoing palliative radiotherapy for advanced breast and head and neck cancer. Results obtained from fine needle aspirate biopsies (FNABs) using this method indicate considerable heterogeneity in hypoxic fraction between tumours. Heterogeneity between 3 aspirates taken from the same 10 tumours immediately following single doses of 3.5 to 5 Gy is now examined. Results indicate that a single fine needle aspirate is reasonably representative for DNA damage and DNA content. However, difficulties were encountered in obtaining an adequate sample of tumour cells after the final radiation treatment. The average hypoxic fraction decreased from 14% after the first dose to 9% after the last dose, and in 3 tumours which could be evaluated after both the first and last fraction, the hypoxic fraction decreased in two but increased in the third. Rejoining of DNA strand breaks was observed between sequential aspirates indicating that pooling of samples for analysis may not be advisable using this method.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman J. D., Dugle D. L., Reuvers A. P., Meeker B. E., Borsa J. Letter: Studies on the radiosensitizing effect of oxygen in Chinese hamster cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Oct;26(4):383–389. doi: 10.1080/09553007414551361. [DOI] [PubMed] [Google Scholar]
- Fairbairn D. W., Olive P. L., O'Neill K. L. The comet assay: a comprehensive review. Mutat Res. 1995 Feb;339(1):37–59. doi: 10.1016/0165-1110(94)00013-3. [DOI] [PubMed] [Google Scholar]
- Lee T. K., Wiley A. L., Jr, Esinhart J. D., Riley R. S., Blackburn L. D. Variations associated with disaggregation methods in DNA flow cytometry. Anal Quant Cytol Histol. 1993 Jun;15(3):195–200. [PubMed] [Google Scholar]
- Ljung B. M., Chew K., Deng G., Matsumura K., Waldman F., Smith H. Fine needle aspiration techniques for the characterization of breast cancers. Cancer. 1994 Aug 1;74(3 Suppl):1000–1005. doi: 10.1002/1097-0142(19940801)74:3+<1000::aid-cncr2820741505>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Olive P. L., Durand R. E. Detection of hypoxic cells in a murine tumor with the use of the comet assay. J Natl Cancer Inst. 1992 May 6;84(9):707–711. doi: 10.1093/jnci/84.9.707. [DOI] [PubMed] [Google Scholar]
- Olive P. L., Durand R. E., Le Riche J., Olivotto I. A., Jackson S. M. Gel electrophoresis of individual cells to quantify hypoxic fraction in human breast cancers. Cancer Res. 1993 Feb 15;53(4):733–736. [PubMed] [Google Scholar]
- Olive P. L. Radiation-induced reoxygenation in the SCCVII murine tumour: evidence for a decrease in oxygen consumption and an increase in tumour perfusion. Radiother Oncol. 1994 Jul;32(1):37–46. doi: 10.1016/0167-8140(94)90447-2. [DOI] [PubMed] [Google Scholar]
- Olive P. L., Vikse C. M., Durand R. E. Hypoxic fractions measured in murine tumors and normal tissues using the comet assay. Int J Radiat Oncol Biol Phys. 1994 Jun 15;29(3):487–491. doi: 10.1016/0360-3016(94)90443-x. [DOI] [PubMed] [Google Scholar]
- Zhang H., Koch C. J., Wallen C. A., Wheeler K. T. Radiation-induced DNA damage in tumors and normal tissues. III. Oxygen dependence of the formation of strand breaks and DNA-protein crosslinks. Radiat Res. 1995 May;142(2):163–168. [PubMed] [Google Scholar]


