Abstract
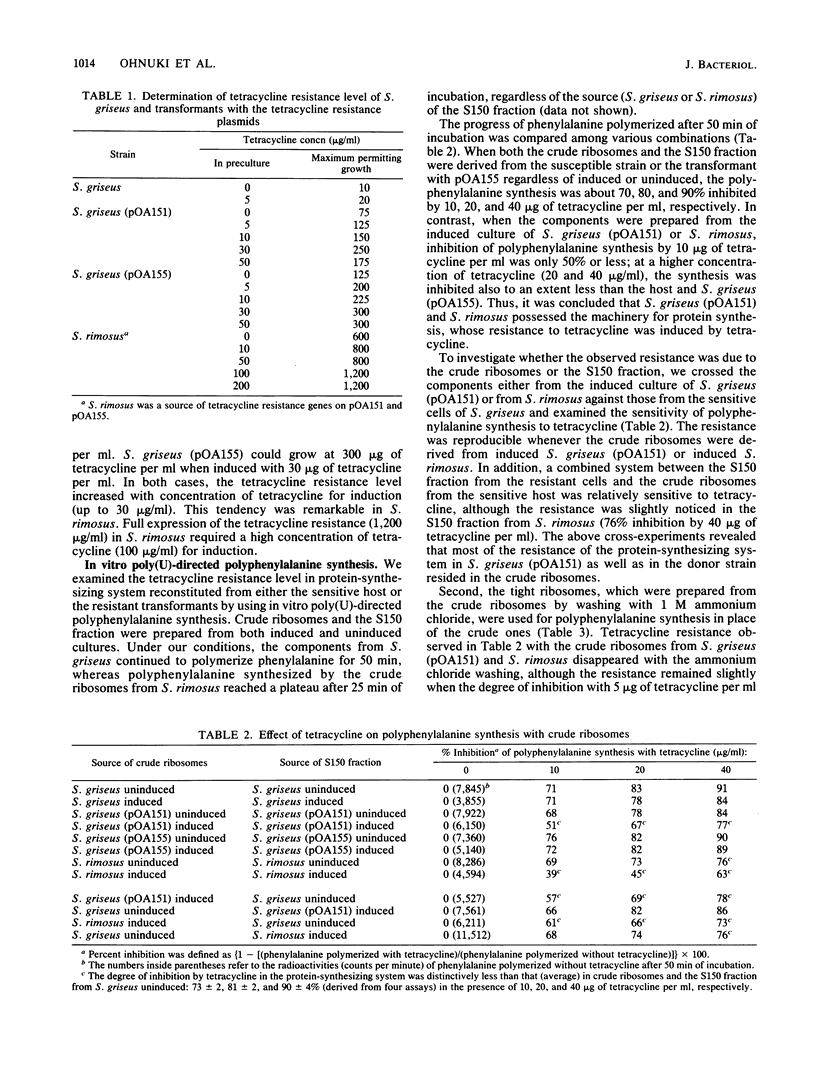
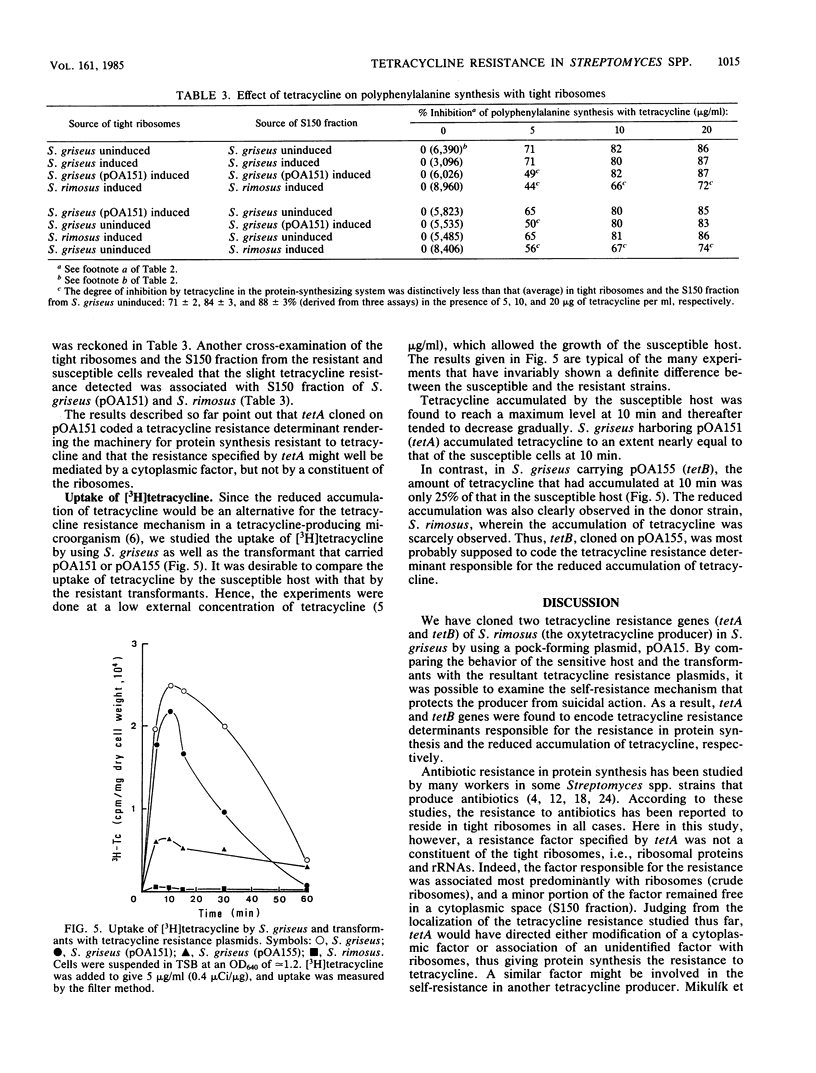
Two tetracycline resistance genes of Streptomyces rimosus, an oxytetracycline producer, were cloned in Streptomyces griseus by using pOA15 as a vector plasmid. Expression of the cloned genes, designated as tetA and tetB was inducible in S. griseus as well as in the donor strain. The tetracycline resistance directed by tetA and tetB was characterized by examining the uptake of tetracycline and in vitro polyphenylalanine synthesis by the sensitive host and transformants with the resultant hybrid plasmids. Polyphenylalanine synthesis with crude ribosomes and the S150 fraction from S. griseus carrying the tetA plasmid was resistant to tetracycline, and, by a cross-test of ribosomes and S150 fraction coming from both the sensitive host and the resistant transformant, the resistance directed by tetA was revealed to reside mainly in crude ribosomes and slightly in the S150 fraction. However, the resistance in the crude ribosomes disappeared when they were washed with 1 M ammonium chloride. These results suggest that tetA specified the tetracycline resistance of the machinery for protein synthesis not through ribosomal subunits, but via an unidentified cytoplasmic factor. In contrast, S. griseus carrying the tetB plasmid accumulated less intracellular tetracycline than did the host, and the protein synthesis by reconstituting the ribosomes and S150 fraction was sensitive to the drug. Therefore, it is conceivable that tetB coded a tetracycline resistance determinant responsible for the reduced accumulation of tetracycline.
Full text
PDF
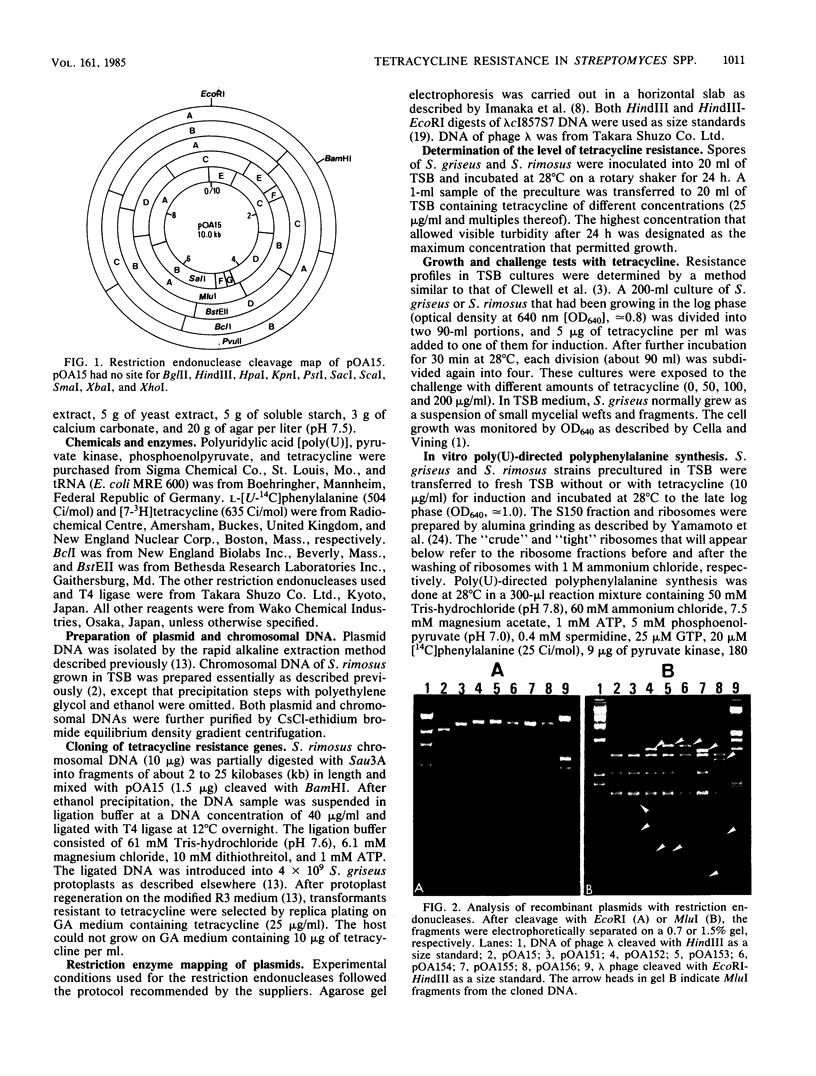
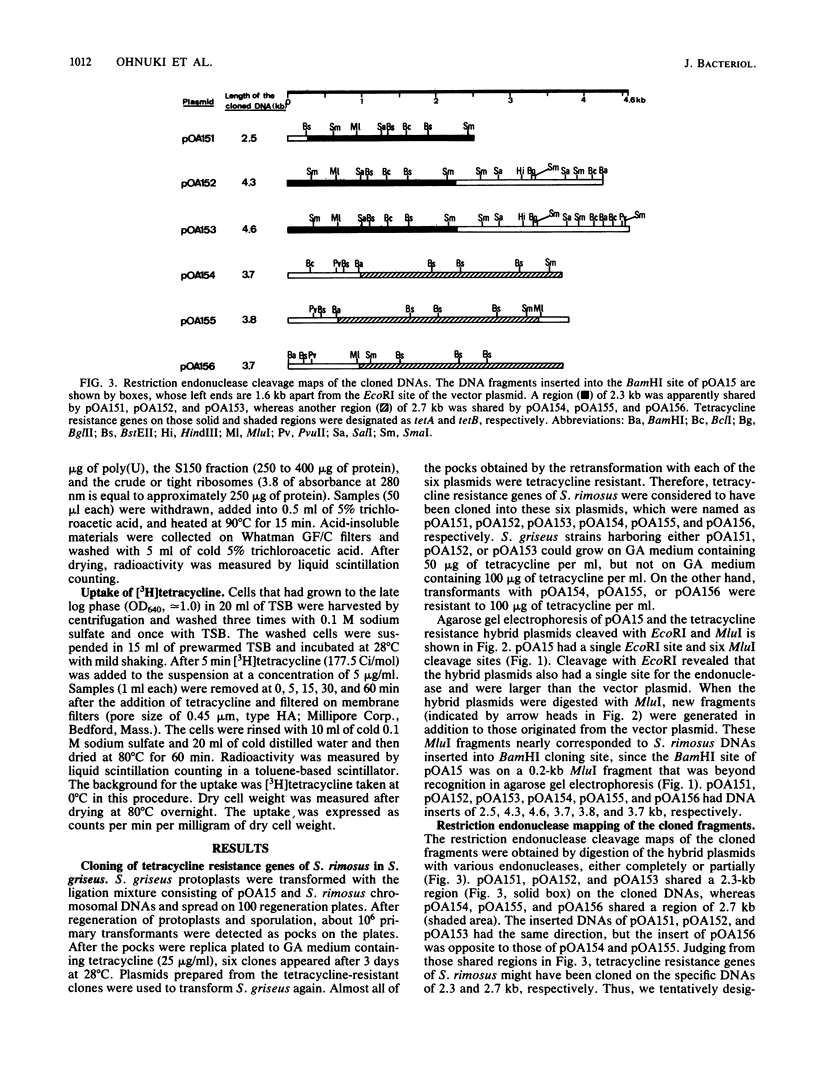
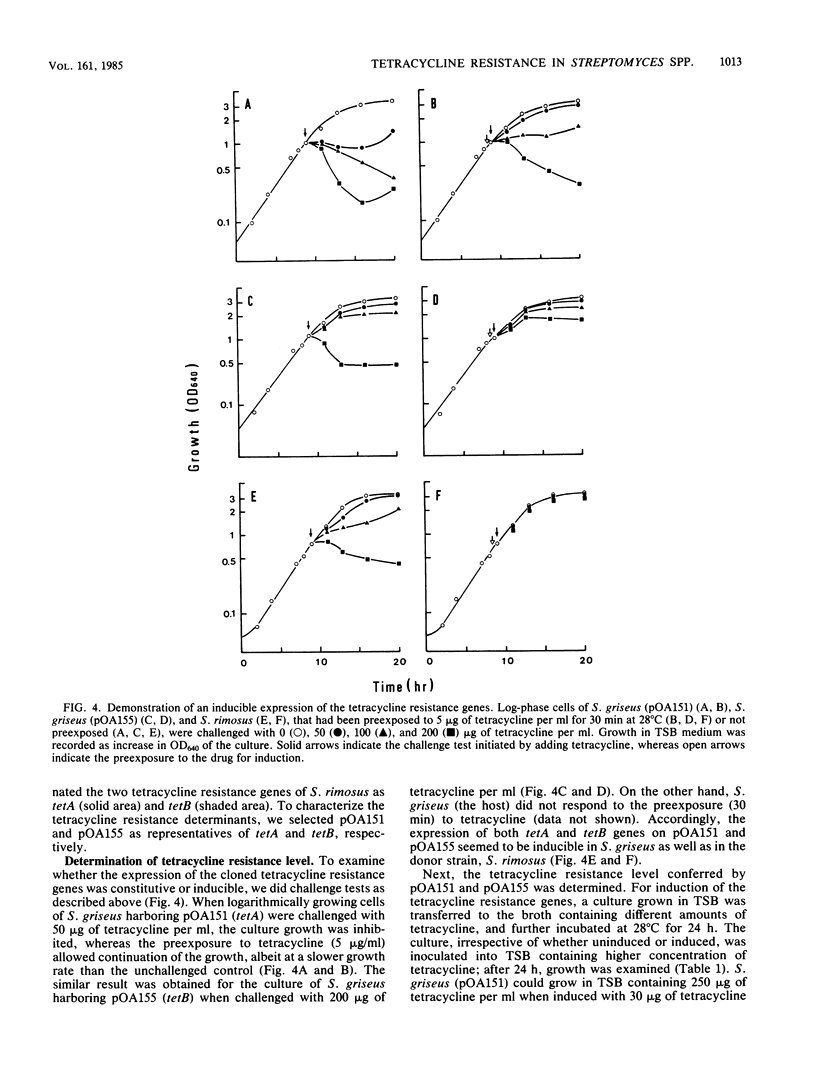

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cella R., Vining L. C. Action of streptomycin on the growth of Streptomyces griseus. Can J Microbiol. 1974 Nov;20(11):1591–1597. doi: 10.1139/m74-246. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Bauer B. Plasmid-determined tetracycline resistance in Streptococcus faecalis: evidence for gene amplification during growth in presence of tetracycline. Proc Natl Acad Sci U S A. 1975 May;72(5):1720–1724. doi: 10.1073/pnas.72.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E., Thompson J. Ribose methylation and resistance to thiostrepton. Nature. 1979 Apr 26;278(5707):859–861. doi: 10.1038/278859a0. [DOI] [PubMed] [Google Scholar]
- Demain A. L. How do antibiotic-producing microorganisms avoid suicide? Ann N Y Acad Sci. 1974 May 10;235(0):601–612. doi: 10.1111/j.1749-6632.1974.tb43294.x. [DOI] [PubMed] [Google Scholar]
- Franklin T. J., Higginson B. Active accumulation of tetracycline by Escherichia coli. Biochem J. 1970 Jan;116(2):287–297. doi: 10.1042/bj1160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Fujii M., Aiba S. Isolation and characterization of antibiotic resistance plasmids from thermophilic bacilli and construction of deletion plasmids. J Bacteriol. 1981 Jun;146(3):1091–1097. doi: 10.1128/jb.146.3.1091-1097.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Levy S. B. Two transport systems for tetracycline in sensitive Escherichia coli: critical role for an initial rapid uptake system insensitive to energy inhibitors. Antimicrob Agents Chemother. 1978 Aug;14(2):201–209. doi: 10.1128/aac.14.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulík K., Jiránová A., Janda I., Weiser J. Susceptibility of ribosomes of the tetracycline-producing strain of Streptomyces aureofaciens to tetracyclines. FEBS Lett. 1983 Feb 7;152(1):125–130. doi: 10.1016/0014-5793(83)80496-7. [DOI] [PubMed] [Google Scholar]
- Mikulík K., Karnetová J., Quyen N., Blumauerová M., Komersová I., Vanek Z. Interaction of tetracycline with protein synthesizing system of Streptomyces aureofaciens. J Antibiot (Tokyo) 1971 Dec;24(12):801–809. doi: 10.7164/antibiotics.24.801. [DOI] [PubMed] [Google Scholar]
- Nakano M. M., Mashiko H., Ogawara H. Cloning of the kanamycin resistance gene from a kanamycin-producing Streptomyces species. J Bacteriol. 1984 Jan;157(1):79–83. doi: 10.1128/jb.157.1.79-83.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuki T., Imanaka T., Aiba S. Isolation and characterization of pock-forming plasmids for Streptomyces griseus from soil actinomycetes. Gene. 1983 Nov;25(1):155–159. doi: 10.1016/0378-1119(83)90178-6. [DOI] [PubMed] [Google Scholar]
- Peters M., Yarus M. Transfer RNA selection at the ribosomal A and P sites. J Mol Biol. 1979 Nov 5;134(3):471–491. doi: 10.1016/0022-2836(79)90364-4. [DOI] [PubMed] [Google Scholar]
- Piwowarski J. M., Shaw P. D. Streptomycin resistance in a streptomycin-producing microorganism. Antimicrob Agents Chemother. 1979 Aug;16(2):176–182. doi: 10.1128/aac.16.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner R. H., Cundliffe E. Resistance to the antibiotics viomycin and capreomycin in the Streptomyces species which produce them. J Gen Microbiol. 1980 Sep;120(1):95–104. doi: 10.1099/00221287-120-1-95. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Boyer H. W. On the nature of tetracycline resistance controlled by the plasmid pSC101. Cell. 1978 Jan;13(1):73–81. doi: 10.1016/0092-8674(78)90139-3. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Skinner R. H., Thompson J., Ward J. M., Hopwood D. A., Cundliffe E. Biochemical characterization of resistance determinants cloned from antibiotic-producing streptomycetes. J Bacteriol. 1982 Aug;151(2):678–685. doi: 10.1128/jb.151.2.678-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. Cloning of antibiotic resistance and nutritional genes in streptomycetes. J Bacteriol. 1982 Aug;151(2):668–677. doi: 10.1128/jb.151.2.668-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vining L. C. Antibiotic tolerance in producer organisms. Adv Appl Microbiol. 1979;25:147–168. doi: 10.1016/s0065-2164(08)70149-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Hotta K., Okami Y., Umezawa H. Self-resistance of a Streptomyces which produces istamycins. J Antibiot (Tokyo) 1981 Jul;34(7):824–829. doi: 10.7164/antibiotics.34.824. [DOI] [PubMed] [Google Scholar]