Abstract
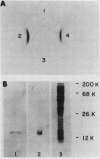
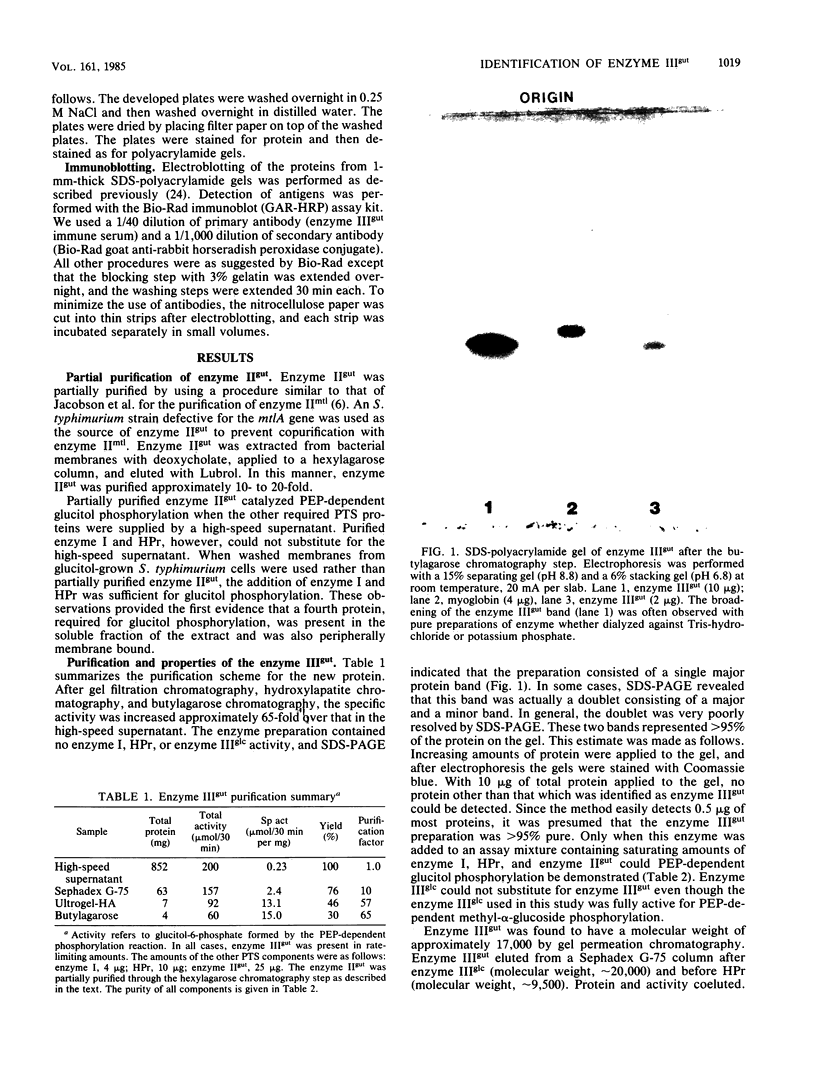
The phosphoenolpyruvate-dependent phosphorylation of glucitol has been shown to require four distinct proteins in Salmonella typhimurium: two general energy-coupling proteins, enzyme I and HPr, and two glucitol-specific proteins, enzyme IIgut and enzyme IIIgut. The enzyme IIgut was solubilized from the membrane and purified about 100-fold, free of the other protein constituents of the phosphotransferase system. Enzyme IIIgut was found in both the soluble and the membrane fractions. The soluble enzyme IIIgut was purified to near homogeneity by gel filtration, hydroxylapatite chromatography, and hydrophobic chromatography on butylagarose. It was sensitive to parital inactivation by trypsin and N-ethylmaleimide, but was stable at 80 degrees C. The protein had an approximate molecular weight of 15,000. It was phosphorylated in the presence of phosphoenolpyruvate, enzyme I, and HPr, and this phosphoprotein was dephosphorylated in the presence of enzyme IIgut and glucitol. Antibodies were raised against enzyme IIIgut. Enzyme IIIglc and enzyme IIIgut exhibited no enzymatic or immunological cross-reactivity. Enzyme IIgut, enzyme IIIgut, and glucitol phosphate dehydrogenase activities were specifically induced by growth in the presence of glucitol. These results serve to characterize the glucitol-specific proteins of the phosphotransferase system in S. typhimurium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyreuther K., Raufuss H., Schrecker O., Hengstenberg W. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus. 1. Amino-acid sequence of the phosphocarrier protein HPr. Eur J Biochem. 1977 May 2;75(1):275–286. doi: 10.1111/j.1432-1033.1977.tb11527.x. [DOI] [PubMed] [Google Scholar]
- Deutscher J., Beyreuther K., Sobek H. M., Stüber K., Hengstenberg W. Phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus: factor IIIlac, a trimeric phospho-carrier protein that also acts as a phase transfer catalyst. Biochemistry. 1982 Sep 28;21(20):4867–4873. doi: 10.1021/bi00263a006. [DOI] [PubMed] [Google Scholar]
- Goudie R. B., Horne C. H., Wilkinson P. C. A simple method for producing antibody specific to a single selected diffusible antigen. Lancet. 1966 Dec 3;2(7475):1224–1226. doi: 10.1016/s0140-6736(66)92305-1. [DOI] [PubMed] [Google Scholar]
- Hays J. B., Simoni R. D., Roseman S. Sugar transport. V. A trimeric lactose-specific phosphocarrier protein of the Staphylococcus aureus phosphotransferase system. J Biol Chem. 1973 Feb 10;248(3):941–956. [PubMed] [Google Scholar]
- Jacobson G. R., Lee C. A., Saier M. H., Jr Purification of the mannitol-specific enzyme II of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1979 Jan 25;254(2):249–252. [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig W. Molecular interactions in the bacterial phosphoenolpyruvate-phosphotransferase system (PTS). J Supramol Struct. 1974;2(5-6):695–814. doi: 10.1002/jss.400020514. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lauppe H. -F., Rau G., Hengstenberg W. Synthese von [32P]phosphoenolpyruvat. FEBS Lett. 1972 Sep 15;25(2):357–357. doi: 10.1016/0014-5793(72)80524-6. [DOI] [PubMed] [Google Scholar]
- Lee C. A., Saier M. H., Jr Mannitol-specific enzyme II of the bacterial phosphotransferase system. III. The nucleotide sequence of the permease gene. J Biol Chem. 1983 Sep 10;258(17):10761–10767. [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols D-mannitol, D-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975 Oct;124(1):26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Nature and properties of hexitol transport systems in Escherichia coli. J Bacteriol. 1975 Oct;124(1):39–47. doi: 10.1128/jb.124.1.39-47.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow N. D., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a glucose-specific phosphocarrier protein (IIIGlc) from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14526–14537. [PubMed] [Google Scholar]
- Osumi T., Saier M. H., Jr Regulation of lactose permease activity by the phosphoenolpyruvate:sugar phosphotransferase system: evidence for direct binding of the glucose-specific enzyme III to the lactose permease. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1457–1461. doi: 10.1073/pnas.79.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarno M. V., Tenn L. G., Desai A., Chin A. M., Grenier F. C., Saier M. H., Jr Genetic evidence for glucitol-specific enzyme III, an essential phosphocarrier protein of the Salmonella typhimurium glucitol phosphotransferase system. J Bacteriol. 1984 Mar;157(3):953–955. doi: 10.1128/jb.157.3.953-955.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Nakazawa T., Hays J. B., Roseman S. Sugar transport. IV. Isolation and characterization of the lactose phosphotransferase system in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):932–940. [PubMed] [Google Scholar]
- Simoni R. D., Smith M. F., Roseman S. Resolution of a staphylococcal phosphotransferase system into four protein components and its relation to sugar transport. Biochem Biophys Res Commun. 1968 Jun 10;31(5):804–811. doi: 10.1016/0006-291x(68)90634-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waygood E. B., Steeves T. Enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system of Escherichia coli. Purification to homogeneity and some properties. Can J Biochem. 1980 Jan;58(1):40–48. doi: 10.1139/o80-006. [DOI] [PubMed] [Google Scholar]
- Wolfson E. B., Sobel M. E., Krulwich T. A. Phosphoenolpyruvate:fructose phosphotransferase activity in whole cells and membrane vesicles of Arthrobacter pyridinolis. Biochim Biophys Acta. 1973 Sep 15;321(1):181–188. doi: 10.1016/0005-2744(73)90072-7. [DOI] [PubMed] [Google Scholar]