Abstract
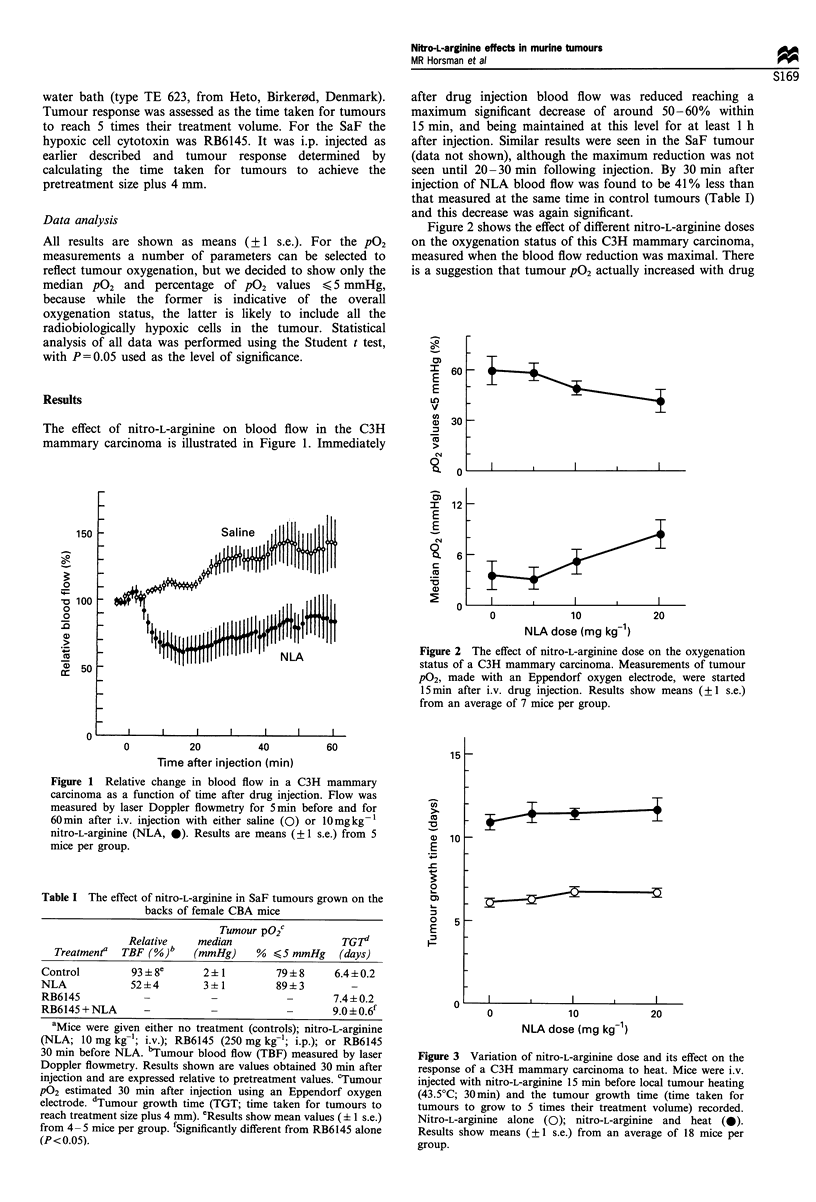
This study was an investigation into the ability of nitro-L-arginine to change blood flow, oxygenation status and the activity of hypoxic cell cytotoxic agents in two different transplanted murine tumours. The tumour models were the C3H mammary carcinoma grown in the feet of female CDF1 mice and the SaF grown on the backs of CBA mice. Treatments were carried out in restrained non-anaesthetised animals when tumours were about 100 to 200 mm3 in size. Blood flow was monitored using laser Doppler flowmetry; oxygen partial pressure (pO2) distributions were obtained with an Eppendorf oxygen electrode; and response to treatment with hyperthermia (43.5 degrees C; 30 min) and RB6145 (250 mg kg-1;i.p.) assessed using a tumour growth delay assay. Nitro-L-arginine (10 mg kg-1; i.v.) significantly reduced blood flow by around 40-60% within 15 min after injection in C3H tumour and by 30 min in the SaF. However, nitro-L-arginine had absolutely no effect on tumour pO2 measured at the time of maximal blood flow reduction in both tumour types. It also failed to enhance the response of the C3H tumour to heat, but did produce a small yet significant increase in the response of the SaF tumour to RB6145.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade S. P., Hart I. R., Piper P. J. Inhibitors of nitric oxide synthase selectively reduce flow in tumor-associated neovasculature. Br J Pharmacol. 1992 Dec;107(4):1092–1095. doi: 10.1111/j.1476-5381.1992.tb13412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. C., Stratford I. J., Bowler J., Adams G. E. Bioreductive drugs and the selective induction of tumour hypoxia. Br J Cancer. 1990 May;61(5):717–721. doi: 10.1038/bjc.1990.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. J., Acker B. The effect of hydralazine on the tumor cytotoxicity of the hypoxic cell cytotoxin RSU-1069: evidence for therapeutic gain. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):579–585. doi: 10.1016/0360-3016(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Denekamp J., Hill S. A., Hobson B. Vascular occlusion and tumour cell death. Eur J Cancer Clin Oncol. 1983 Feb;19(2):271–275. doi: 10.1016/0277-5379(83)90426-1. [DOI] [PubMed] [Google Scholar]
- Dworkin M. J., Carnochan P., Allen-Mersh T. G. Nitric oxide inhibition sustains vasopressin-induced vasoconstriction. Br J Cancer. 1995 May;71(5):942–944. doi: 10.1038/bjc.1995.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerweck L. E., Nygaard T. G., Burlett M. Response of cells to hyperthermia under acute and chronic hypoxic conditions. Cancer Res. 1979 Mar;39(3):966–972. [PubMed] [Google Scholar]
- Hill S. A., Chaplin D. J. The effect of nicotinamide on microregional blood flow within tumours assessed using laser Doppler probes. Acta Oncol. 1995;34(3):401–404. doi: 10.3109/02841869509093997. [DOI] [PubMed] [Google Scholar]
- Hill S. A., Denekamp J. The effect of vascular occlusion on the thermal sensitization of a mouse tumour. Br J Radiol. 1978 Dec;51(612):997–1002. doi: 10.1259/0007-1285-51-612-997. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Christensen K. L., Overgaard J. Hydralazine-induced enhancement of hyperthermic damage in a C3H mammary carcinoma in vivo. Int J Hyperthermia. 1989 Mar-Apr;5(2):123–136. doi: 10.3109/02656738909140442. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Christensen K. L., Overgaard J. Relationship between the hydralazine-induced changes in murine tumor blood supply and mouse blood pressure. Int J Radiat Oncol Biol Phys. 1992;22(3):455–458. doi: 10.1016/0360-3016(92)90852-9. [DOI] [PubMed] [Google Scholar]
- Kallinowski F., Zander R., Hoeckel M., Vaupel P. Tumor tissue oxygenation as evaluated by computerized-pO2-histography. Int J Radiat Oncol Biol Phys. 1990 Oct;19(4):953–961. doi: 10.1016/0360-3016(90)90018-f. [DOI] [PubMed] [Google Scholar]
- Kalmus J., Okunieff P., Vaupel P. Dose-dependent effects of hydralazine on microcirculatory function and hyperthermic response of murine FSall tumors. Cancer Res. 1990 Jan 1;50(1):15–19. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Overgaard J., Bichel P. The influence of hypoxia and acidity on the hyperthermic response of malignant cells in vitro. Radiology. 1977 May;123(2):511–514. doi: 10.1148/123.2.511. [DOI] [PubMed] [Google Scholar]
- Overgaard J., Nielsen O. S. The role of tissue environmental factors on the kinetics and morphology of tumor cells exposed to hyperthermia. Ann N Y Acad Sci. 1980;335:254–280. doi: 10.1111/j.1749-6632.1980.tb50753.x. [DOI] [PubMed] [Google Scholar]
- Overgaard J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int J Radiat Oncol Biol Phys. 1980 Nov;6(11):1507–1517. doi: 10.1016/0360-3016(80)90008-5. [DOI] [PubMed] [Google Scholar]
- Tozer G. M., Prise V. E., Bell K. M. The influence of nitric oxide on tumour vascular tone. Acta Oncol. 1995;34(3):373–377. doi: 10.3109/02841869509093992. [DOI] [PubMed] [Google Scholar]
- Wood P. J., Sansom J. M., Butler S. A., Stratford I. J., Cole S. M., Szabo C., Thiemermann C., Adams G. E. Induction of hypoxia in experimental murine tumors by the nitric oxide synthase inhibitor, NG-nitro-L-arginine. Cancer Res. 1994 Dec 15;54(24):6458–6463. [PubMed] [Google Scholar]
- Wood P. J., Sansom J. M., Stratford I. J., Adams G. E., Szabo C., Thiemermann C., Vane J. R. Modification of metabolism of transplantable and spontaneous murine tumors by the nitric oxide synthase inhibitor, nitro-L-arginine. Int J Radiat Oncol Biol Phys. 1994 Jun 15;29(3):443–447. doi: 10.1016/0360-3016(94)90435-9. [DOI] [PubMed] [Google Scholar]


