Abstract
It is known that radiation therapy results in some form of damage to the microcirculation. In support of this view, we found that capillary endothelial cells (EC) treated with X-rays (8 Gy) were defective in their ability to recover a denuded area. A scrape wound of 2 mm width was produced in monolayers 30 min after X-ray or sham treatment. After 48 h, the number of cells migrating into each of five successive 125 microns zones from both sides of the original wound were determined. Greater numbers of sham-treated EC entered zones 3 and 4, compared with irradiated cultures, and only sham-treated EC entered the most distant zone 5. We examined actin fibre orientation within migrating irradiated and sham-treated EC using 2-(D-2-aminobutanoic acid)-7-(N6-((((3,6-bis(dimethylamino)xanthylium-9-yl) carboxyphenyl) amino)thioxomethyl)-L-lysine), chloride (NBD)-phalloidin, immunofluorescent microscopy and computer image analysis. After 48 h, sham-treated, but not irradiated EC, contained actin which was orientated perpendicular to the original wound edge. After 6-9 days, only sham-treated EC closed the wounds. Tempol (4 hydroxy-2,2,6,6-tetra methylpiperidine-1-oxyl)(0.5 or 2 mM)), included in the media during irradiation, prevented this wound healing delay, when measured within the first 24 h. In conclusion, radiation treatment of capillary EC results in a wound healing defect. This defect appears to be related to the EC's inability to realign actin. Tempol protects EC from exhibiting a wound healing delay.
Full text
PDF
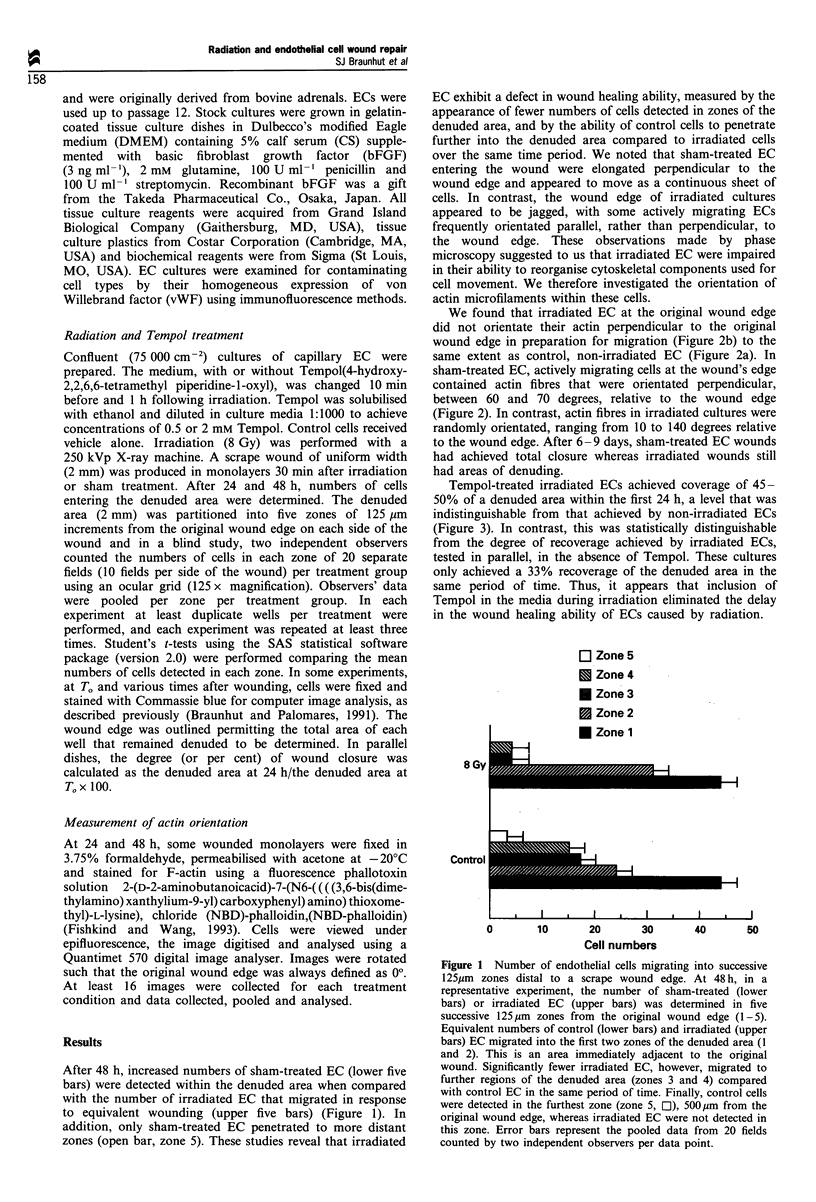
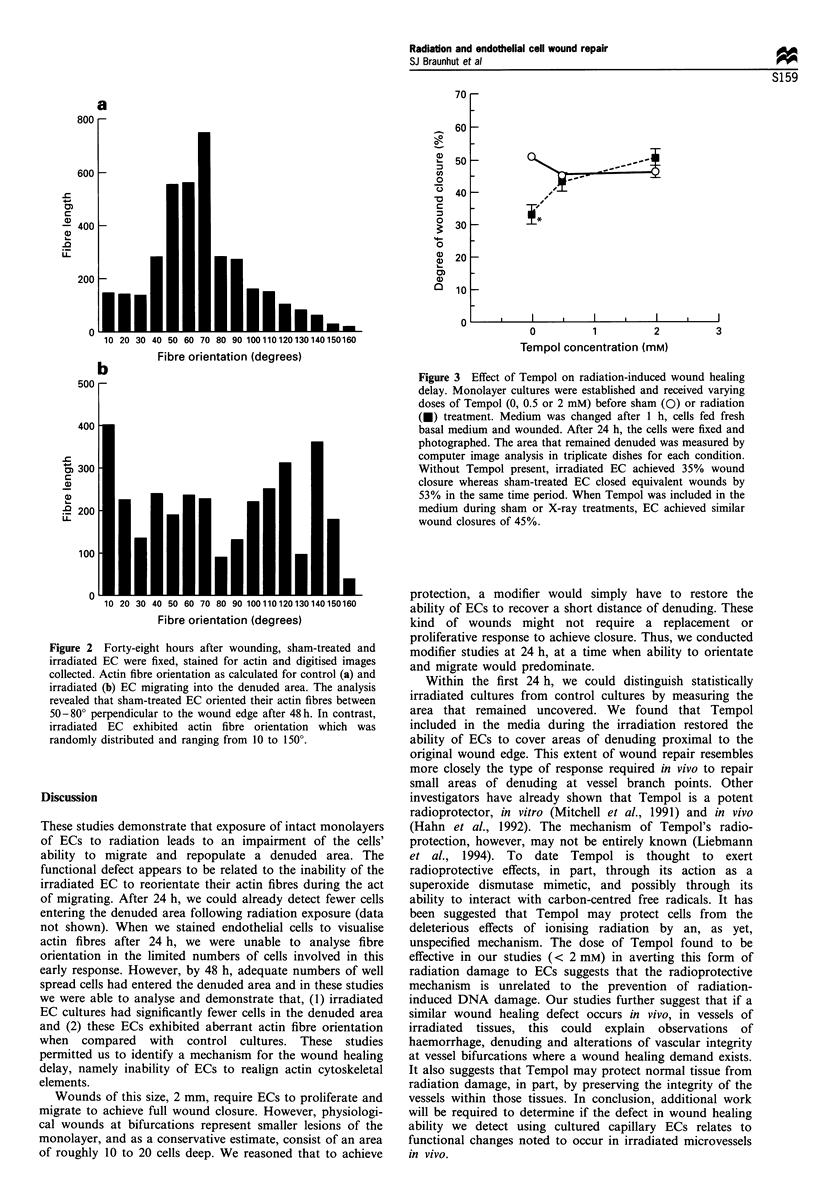

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Endothelial injury and repair in radiation-induced pulmonary fibrosis. Am J Pathol. 1983 Aug;112(2):224–230. [PMC free article] [PubMed] [Google Scholar]
- Braunhut S. J., Palomares M. Modulation of endothelial cell shape and growth by retinoids. Microvasc Res. 1991 Jan;41(1):47–62. doi: 10.1016/0026-2862(91)90007-x. [DOI] [PubMed] [Google Scholar]
- Denekamp J., Hobson B. Endothelial-cell proliferation in experimental tumours. Br J Cancer. 1982 Nov;46(5):711–720. doi: 10.1038/bjc.1982.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engerman R. L., Pfaffenbach D., Davis M. D. Cell turnover of capillaries. Lab Invest. 1967 Dec;17(6):738–743. [PubMed] [Google Scholar]
- Fajardo L. F., Brown J. M., Glatstein E. Glomerular and juxta-glomerular lesions in radiation nephropathy. Radiat Res. 1976 Oct;68(1):177–183. [PubMed] [Google Scholar]
- Fishkind D. J., Wang Y. L. Orientation and three-dimensional organization of actin filaments in dividing cultured cells. J Cell Biol. 1993 Nov;123(4):837–848. doi: 10.1083/jcb.123.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S. M., Tochner Z., Krishna C. M., Glass J., Wilson L., Samuni A., Sprague M., Venzon D., Glatstein E., Mitchell J. B. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992 Apr 1;52(7):1750–1753. [PubMed] [Google Scholar]
- Hopewell J. W., Campling D., Calvo W., Reinhold H. S., Wilkinson J. H., Yeung T. K. Vascular irradiation damage: its cellular basis and likely consequences. Br J Cancer Suppl. 1986;7:181–191. [PMC free article] [PubMed] [Google Scholar]
- Jordan S. W., Key C. R., Gomez L. S., Agnew J., Barton S. L. Late effects of radiation on the mouse kidney. Exp Mol Pathol. 1978 Aug;29(1):115–129. doi: 10.1016/0014-4800(78)90031-x. [DOI] [PubMed] [Google Scholar]
- Kantak S. S., Diglio C. A., Onoda J. M. Low dose radiation-induced endothelial cell retraction. Int J Radiat Biol. 1993 Sep;64(3):319–328. doi: 10.1080/09553009314551471. [DOI] [PubMed] [Google Scholar]
- Krishnan L., Krishnan E. C., Jewell W. R. Immediate effect of irradiation on microvasculature. Int J Radiat Oncol Biol Phys. 1988 Jul;15(1):147–150. doi: 10.1016/0360-3016(88)90359-8. [DOI] [PubMed] [Google Scholar]
- Lampugnani M. G., Resnati M., Dejana E., Marchisio P. C. The role of integrins in the maintenance of endothelial monolayer integrity. J Cell Biol. 1991 Feb;112(3):479–490. doi: 10.1083/jcb.112.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauk S., Trott K. R. Endothelial cell proliferation in the rat heart following local heart irradiation. Int J Radiat Biol. 1990 May;57(5):1017–1030. doi: 10.1080/09553009014551131. [DOI] [PubMed] [Google Scholar]
- Liebmann J., DeLuca A. M., Coffin D., Keefer L. K., Venzon D., Wink D. A., Mitchell J. B. In vivo radiation protection by nitric oxide modulation. Cancer Res. 1994 Jul 1;54(13):3365–3368. [PubMed] [Google Scholar]
- Mitchell J. B., DeGraff W., Kaufman D., Krishna M. C., Samuni A., Finkelstein E., Ahn M. S., Hahn S. M., Gamson J., Russo A. Inhibition of oxygen-dependent radiation-induced damage by the nitroxide superoxide dismutase mimic, tempol. Arch Biochem Biophys. 1991 Aug 15;289(1):62–70. doi: 10.1016/0003-9861(91)90442-l. [DOI] [PubMed] [Google Scholar]
- Pepper M. S., Sappino A. P., Stöcklin R., Montesano R., Orci L., Vassalli J. D. Upregulation of urokinase receptor expression on migrating endothelial cells. J Cell Biol. 1993 Aug;122(3):673–684. doi: 10.1083/jcb.122.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]


