Abstract
It is well known that low levels of tissue oxygen (pO2) protect tumour cells from ionising radiation and some chemotherapeutic agents. Thus, numerous studies have been aimed at developing methods to measure tissue oxygenation. An initial discussion of some of the traditional methods for measuring oxygenation is included, followed by a discussion of magnetic resonance spectroscopy (MRS) and magnetic resonance imaging (MRI) methods for measuring tumour and normal tissue oxygenation. The latter methods are of interest because of the non-invasive nature of magnetic resonance (MR). Some of the MR methods described herein include: 31P MRS, 1H MRS and MRI, and 19F MRS and MRI. Each method is detailed, including a brief assessment of its ability to measure tumour oxygenation and its potential for clinical application.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacić G., Liu K. J., O'Hara J. A., Harris R. D., Szybinski K., Goda F., Swartz H. M. Oxygen tension in a murine tumor: a combined EPR and MRI study. Magn Reson Med. 1993 Nov;30(5):568–572. doi: 10.1002/mrm.1910300507. [DOI] [PubMed] [Google Scholar]
- Brown J. M. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979 Aug;52(620):650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- Chance B., Barlow C., Nakase Y., Takeda H., Mayevsky A., Fischetti R., Graham N., Sorge J. Heterogeneity of oxygen delivery in normoxic and hypoxic states: a fluorometer study. Am J Physiol. 1978 Dec;235(6):H809–H820. doi: 10.1152/ajpheart.1978.235.6.H809. [DOI] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr, Clark B. J., Maris J., Kent J., Nioka S., Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8384–8388. doi: 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. D., Franko A. J., Sharplin J. A marker for hypoxic cells in tumours with potential clinical applicability. Br J Cancer. 1981 Apr;43(4):546–550. doi: 10.1038/bjc.1981.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardzinski B. J., Sotak C. H. Rapid tissue oxygen tension mapping using 19F inversion-recovery echo-planar imaging of perfluoro-15-crown-5-ether. Magn Reson Med. 1994 Jul;32(1):88–97. doi: 10.1002/mrm.1910320112. [DOI] [PubMed] [Google Scholar]
- Evelhoch J. L., Sapareto S. A., Nussbaum G. H., Ackerman J. J. Correlations between 31P NMR spectroscopy and 15O perfusion measurements in the RIF-1 murine tumor in vivo. Radiat Res. 1986 Apr;106(1):122–131. [PubMed] [Google Scholar]
- Gerweck L. E., Koutcher J., Zaidi S. T. Energy status parameters, hypoxia fraction and radiocurability across tumor types. Acta Oncol. 1995;34(3):335–338. doi: 10.3109/02841869509093985. [DOI] [PubMed] [Google Scholar]
- Goda F., Liu K. J., Walczak T., O'Hara J. A., Jiang J., Swartz H. M. In vivo oximetry using EPR and India ink. Magn Reson Med. 1995 Feb;33(2):237–245. doi: 10.1002/mrm.1910330214. [DOI] [PubMed] [Google Scholar]
- Goda F., O'Hara J. A., Rhodes E. S., Liu K. J., Dunn J. F., Bacic G., Swartz H. M. Changes of oxygen tension in experimental tumors after a single dose of X-ray irradiation. Cancer Res. 1995 Jun 1;55(11):2249–2252. [PubMed] [Google Scholar]
- Jin G. Y., Li S. J., Moulder J. E., Raleigh J. A. Dynamic measurements of hexafluoromisonidazole (CCI-103F) retention in mouse tumours by 1H/19F magnetic resonance spectroscopy. Int J Radiat Biol. 1990 Dec;58(6):1025–1034. doi: 10.1080/09553009014552331. [DOI] [PubMed] [Google Scholar]
- Jue T., Anderson S. 1H NMR observation of tissue myoglobin: an indicator of cellular oxygenation in vivo. Magn Reson Med. 1990 Mar;13(3):524–528. doi: 10.1002/mrm.1910130322. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977 Dec 23;198(4323):1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- Karczmar G. S., River J. N., Li J., Vijayakumar S., Goldman Z., Lewis M. Z. Effects of hyperoxia on T2* and resonance frequency weighted magnetic resonance images of rodent tumours. NMR Biomed. 1994 Mar;7(1-2):3–11. doi: 10.1002/nbm.1940070103. [DOI] [PubMed] [Google Scholar]
- Kreutzer U., Wang D. S., Jue T. Observing the 1H NMR signal of the myoglobin Val-E11 in myocardium: an index of cellular oxygenation. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4731–4733. doi: 10.1073/pnas.89.10.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman VYu, River J. N., Lewis M. Z., Lubich L. M., Karczmar G. S. Changes in T2*-weighted images during hyperoxia differentiate tumors from normal tissue. Magn Reson Med. 1995 Mar;33(3):318–325. doi: 10.1002/mrm.1910330306. [DOI] [PubMed] [Google Scholar]
- Kuppusamy P., Wang P., Zweier J. L. Three-dimensional spatial EPR imaging of the rat heart. Magn Reson Med. 1995 Jul;34(1):99–105. doi: 10.1002/mrm.1910340115. [DOI] [PubMed] [Google Scholar]
- Kwock L., Gill M., McMurry H. L., Beckman W., Raleigh J. A., Joseph A. P. Evaluation of a fluorinated 2-nitroimidazole binding to hypoxic cells in tumor-bearing rats by 19F magnetic resonance spectroscopy and immunohistochemistry. Radiat Res. 1992 Jan;129(1):71–78. [PubMed] [Google Scholar]
- Kwong K. K., Belliveau J. W., Chesler D. A., Goldberg I. E., Weisskoff R. M., Poncelet B. P., Kennedy D. N., Hoppel B. E., Cohen M. S., Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell R. J., Workman P., Griffiths J. R. Demonstration of tumor-selective retention of fluorinated nitroimidazole probes by 19F magnetic resonance spectroscopy in vivo. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):925–929. doi: 10.1016/0360-3016(89)90888-2. [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W., Schaefer C., Walenta S., Rofstad E. K., Fenton B. M., Sutherland R. M. Assessment of tumor energy and oxygenation status by bioluminescence, nuclear magnetic resonance spectroscopy, and cryospectrophotometry. Cancer Res. 1990 Mar 15;50(6):1681–1685. [PubMed] [Google Scholar]
- Ogawa S., Lee T. M., Nayak A. S., Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990 Apr;14(1):68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Raleigh J. A., Franko A. J., Kelly D. A., Trimble L. A., Allen P. S. Development of an in vivo 19F magnetic resonance method for measuring oxygen deficiency in tumors. Magn Reson Med. 1991 Dec;22(2):451–466. doi: 10.1002/mrm.1910220253. [DOI] [PubMed] [Google Scholar]
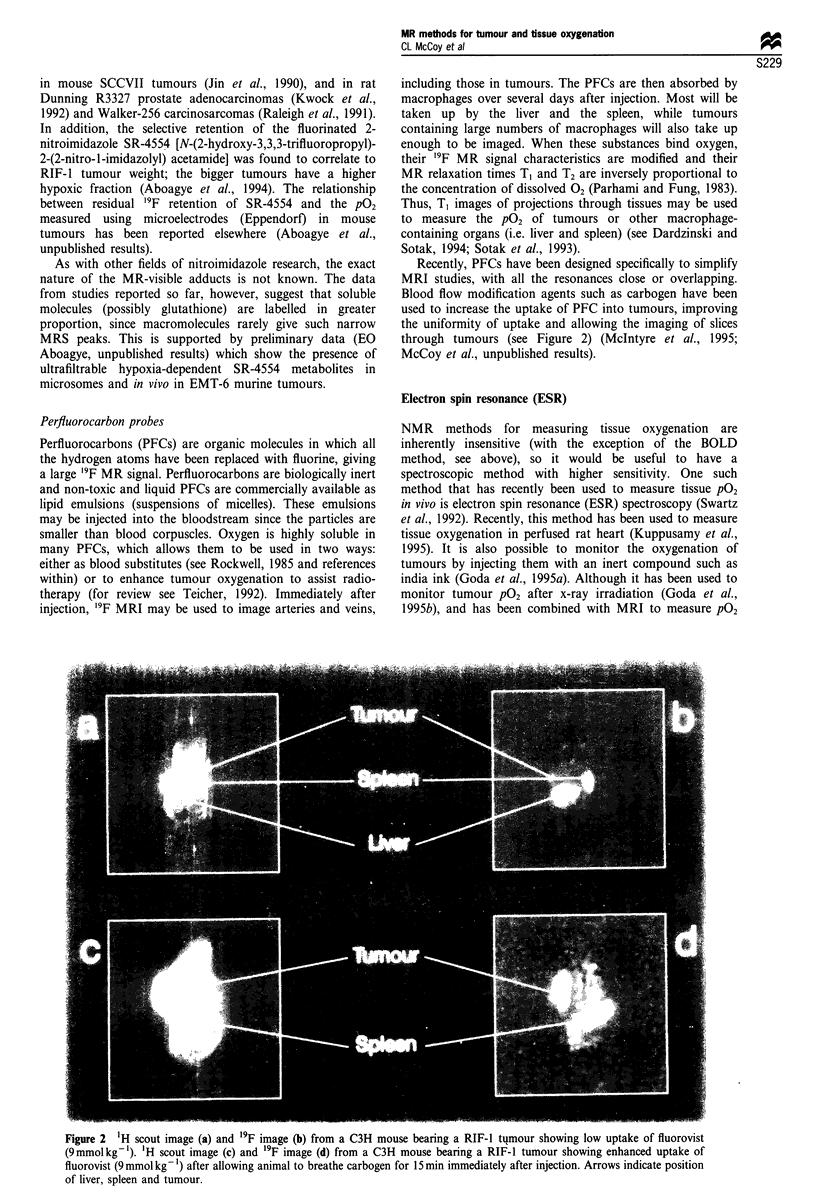
- Robinson S. P., Howe F. A., Griffiths J. R. Noninvasive monitoring of carbogen-induced changes in tumor blood flow and oxygenation by functional magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 1995 Nov 1;33(4):855–859. doi: 10.1016/0360-3016(95)00072-1. [DOI] [PubMed] [Google Scholar]
- Rockwell S. Use of a perfluorochemical emulsion to improve oxygenation in a solid tumor. Int J Radiat Oncol Biol Phys. 1985 Jan;11(1):97–103. doi: 10.1016/0360-3016(85)90367-0. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K., DeMuth P., Fenton B. M., Sutherland R. M. 31P nuclear magnetic resonance spectroscopy studies of tumor energy metabolism and its relationship to intracapillary oxyhemoglobin saturation status and tumor hypoxia. Cancer Res. 1988 Oct 1;48(19):5440–5446. [PubMed] [Google Scholar]
- Rofstad E. K., Howell R. L., DeMuth P., Ceckler T. L., Sutherland R. M. 31P NMR spectroscopy in vivo of two murine tumor lines with widely different fractions of radiobiologically hypoxic cells. Int J Radiat Biol. 1988 Oct;54(4):635–649. doi: 10.1080/09553008814552071. [DOI] [PubMed] [Google Scholar]
- Shungu D. C., Bhujwalla Z. M., Wehrle J. P., Glickson J. D. 1H NMR spectroscopy of subcutaneous tumors in mice: preliminary studies of effects of growth, chemotherapy and blood flow reduction. NMR Biomed. 1992 Sep-Oct;5(5):296–302. doi: 10.1002/nbm.1940050517. [DOI] [PubMed] [Google Scholar]
- Sostman H. D., Rockwell S., Sylvia A. L., Madwed D., Cofer G., Charles H. C., Negro-Vilar R., Moore D. Evaluation of BA1112 rhabdomyosarcoma oxygenation with microelectrodes, optical spectrophotometry, radiosensitivity, and magnetic resonance spectroscopy. Magn Reson Med. 1991 Aug;20(2):253–267. doi: 10.1002/mrm.1910200208. [DOI] [PubMed] [Google Scholar]
- Sotak C. H., Hees P. S., Huang H. N., Hung M. H., Krespan C. G., Raynolds S. A new perfluorocarbon for use in fluorine-19 magnetic resonance imaging and spectroscopy. Magn Reson Med. 1993 Feb;29(2):188–195. doi: 10.1002/mrm.1910290206. [DOI] [PubMed] [Google Scholar]
- Swartz H. M., Boyer S., Brown D., Chang K., Gast P., Glockner J. F., Hu H., Liu K. J., Moussavi M., Nilges M. The use of EPR for the measurement of the concentration of oxygen in vivo in tissues under physiologically pertinent conditions and concentrations. Adv Exp Med Biol. 1992;317:221–228. doi: 10.1007/978-1-4615-3428-0_23. [DOI] [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher B. A. Use of perfluorochemical emulsions in cancer therapy. Biomater Artif Cells Immobilization Biotechnol. 1992;20(2-4):875–882. doi: 10.3109/10731199209119734. [DOI] [PubMed] [Google Scholar]
- Tozer G. M., Griffiths J. R. The contribution made by cell death and oxygenation to 31P MRS observations of tumour energy metabolism. NMR Biomed. 1992 Sep-Oct;5(5):279–289. doi: 10.1002/nbm.1940050515. [DOI] [PubMed] [Google Scholar]
- Vaupel P. W., Frinak S., Bicher H. I. Heterogeneous oxygen partial pressure and pH distribution in C3H mouse mammary adenocarcinoma. Cancer Res. 1981 May;41(5):2008–2013. [PubMed] [Google Scholar]
- Vaupel P., Okunieff P., Kallinowski F., Neuringer L. J. Correlations between 31P-NMR spectroscopy and tissue O2 tension measurements in a murine fibrosarcoma. Radiat Res. 1989 Dec;120(3):477–493. [PubMed] [Google Scholar]
- Vaupel P., Schaefer C., Okunieff P. Intracellular acidosis in murine fibrosarcomas coincides with ATP depletion, hypoxia, and high levels of lactate and total Pi. NMR Biomed. 1994 May;7(3):128–136. doi: 10.1002/nbm.1940070305. [DOI] [PubMed] [Google Scholar]
- Vink R. Nuclear magnetic resonance spectroscopy and the study of tissue oxygen metabolism: a review. Adv Exp Med Biol. 1992;316:187–193. doi: 10.1007/978-1-4615-3404-4_22. [DOI] [PubMed] [Google Scholar]
- Wang D. J., Nioka S., Wang Z., Leigh J. S., Chance B. NMR visibility studies of N-delta proton of proximal histidine in deoxyhemoglobin in lysed and intact red cells. Magn Reson Med. 1993 Dec;30(6):759–763. doi: 10.1002/mrm.1910300616. [DOI] [PubMed] [Google Scholar]
- Wang Z., Wang D. J., Noyszewski E. A., Bogdan A. R., Haselgrove J. C., Reddy R., Zimmerman R. A., Leigh J. S. Sensitivity of in vivo MRS of the N-delta proton in proximal histidine of deoxymyoglobin. Magn Reson Med. 1992 Oct;27(2):362–367. doi: 10.1002/mrm.1910270217. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Cerniglia G. J. Localization of tumors and evaluation of their state of oxygenation by phosphorescence imaging. Cancer Res. 1992 Jul 15;52(14):3988–3993. [PubMed] [Google Scholar]
- Workman P., Brown J. M. Structure-pharmacokinetic relationships for misonidazole analogues in mice. Cancer Chemother Pharmacol. 1981;6(1):39–49. doi: 10.1007/BF00253009. [DOI] [PubMed] [Google Scholar]
- Workman P., Maxwell R. J., Griffiths J. R. Non-invasive MRS in new anticancer drug development. NMR Biomed. 1992 Sep-Oct;5(5):270–272. doi: 10.1002/nbm.1940050513. [DOI] [PubMed] [Google Scholar]