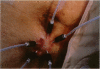
Abstract
A multichannel laser Doppler system has been used to measure microregional fluctuations in perfusion in the HT29 human tumour xenograft and in patients with advanced malignant disease. A comparison is made with previously obtained data for the SaF, a transplantable murine tumour. The 300 microns diameter probes recorded fluctuations in erythrocyte flux in tumour microregions with an estimated volume of 10(-2) mm3. Of the 66 human tumour microregions sampled, 26% showed a change in erythrocyte flux by a factor of 2 or more over the 60 min measurement period, compared with 37% of HT29 and 48% of SaF microregions. In each of the studies more than 50% of changes were completed within 20 min, although slower changes were more common in the human tumours than in the experimental systems. Within the 1 h monitoring period at least 30% of the changes were reversed (human tumours 30%, HT29 45%, SaF 31%). These findings demonstrate that microregional changes in erythrocyte flux, consistent with transient, perfusion-driven changes in oxygenation, are a feature of human malignancies as well as experimental transplanted tumours.
Full text
PDF
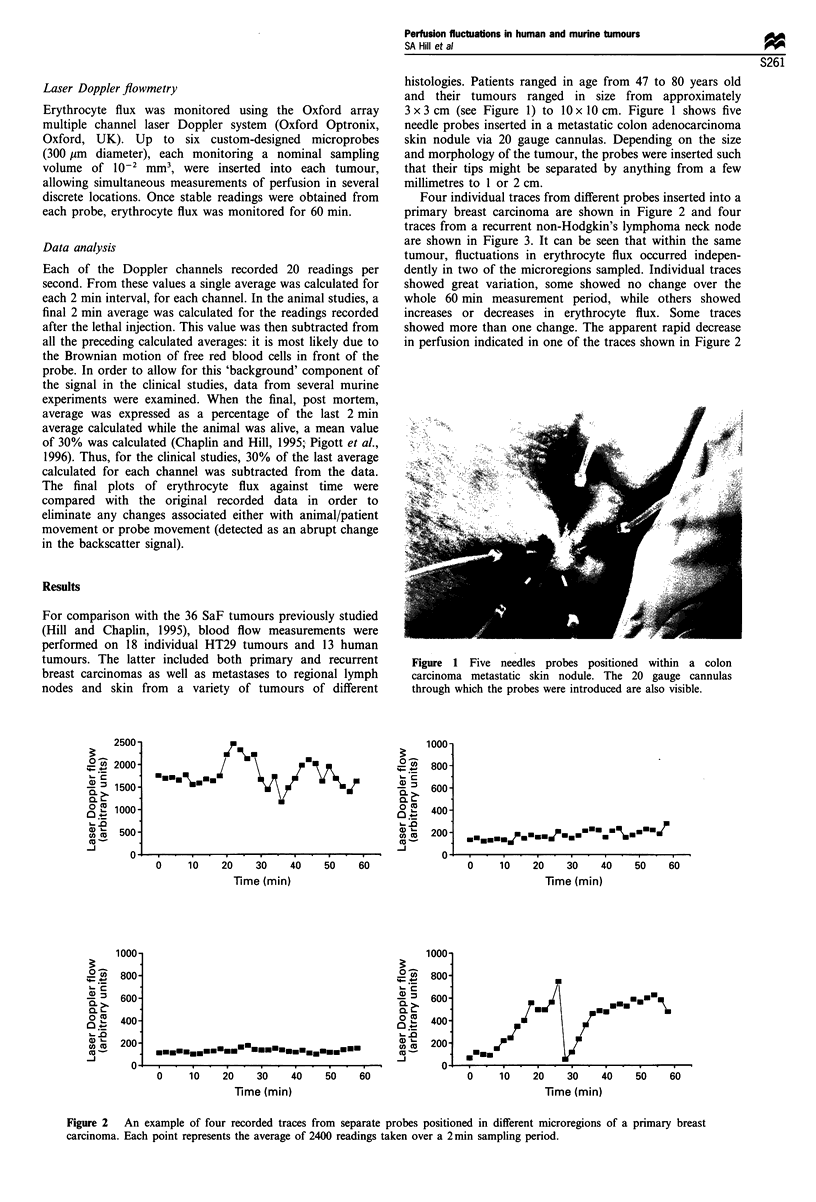
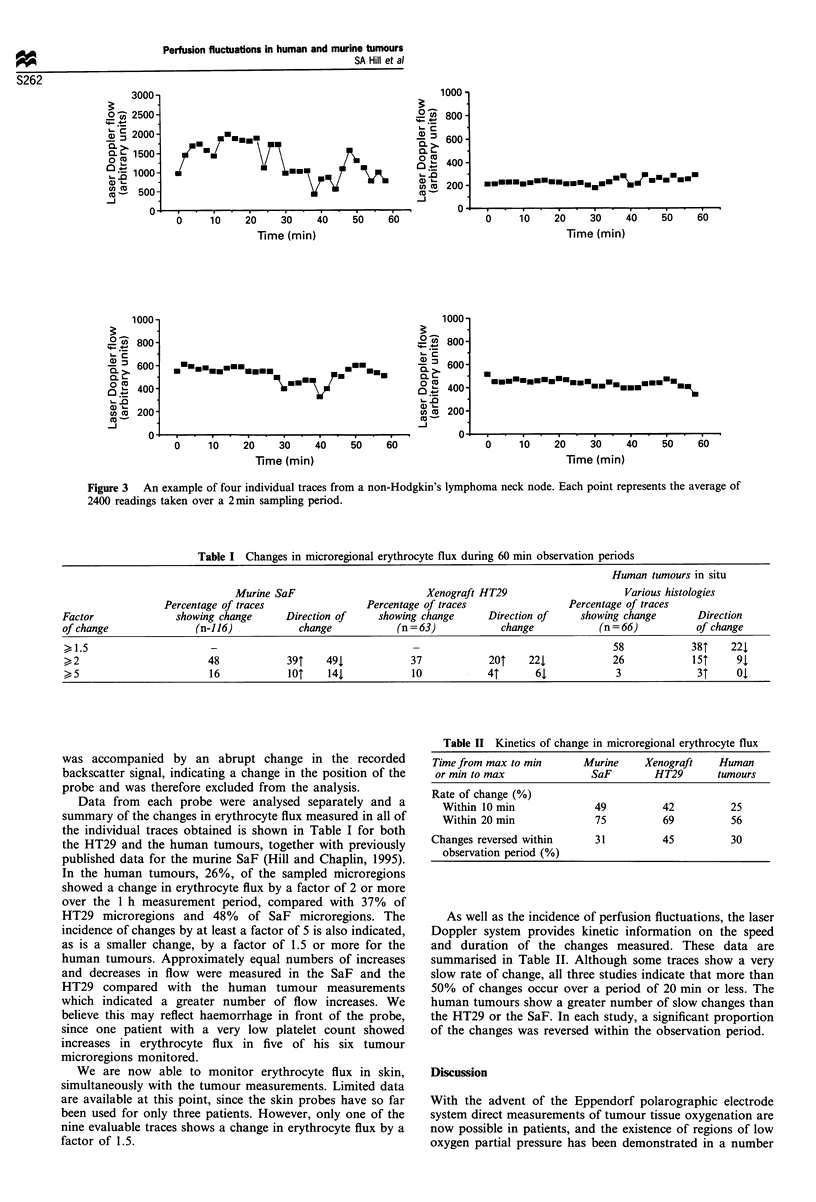

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. M. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979 Aug;52(620):650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Hill S. A. Temporal heterogeneity in microregional erythrocyte flux in experimental solid tumours. Br J Cancer. 1995 Jun;71(6):1210–1213. doi: 10.1038/bjc.1995.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. J., Horsman M. R., Trotter M. J. Effect of nicotinamide on the microregional heterogeneity of oxygen delivery within a murine tumor. J Natl Cancer Inst. 1990 Apr 18;82(8):672–676. doi: 10.1093/jnci/82.8.672. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Olive P. L., Durand R. E. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Res. 1987 Jan 15;47(2):597–601. [PubMed] [Google Scholar]
- Dische S. Chemical sensitizers for hypoxic cells: a decade of experience in clinical radiotherapy. Radiother Oncol. 1985 Feb;3(2):97–115. doi: 10.1016/s0167-8140(85)80015-3. [DOI] [PubMed] [Google Scholar]
- Henk J. M. Does hyperbaric oxygen have a future in radiation therapy? Int J Radiat Oncol Biol Phys. 1981 Aug;7(8):1125–1128. doi: 10.1016/0360-3016(81)90173-5. [DOI] [PubMed] [Google Scholar]
- Hill S. A., Chaplin D. J. The effect of nicotinamide on microregional blood flow within tumours assessed using laser Doppler probes. Acta Oncol. 1995;34(3):401–404. doi: 10.3109/02841869509093997. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Brown J. M., Hirst V. K., Lemmon M. J., Wood P. J., Dunphy E. P., Overgaard J. Mechanism of action of the selective tumor radiosensitizer nicotinamide. Int J Radiat Oncol Biol Phys. 1988 Sep;15(3):685–690. doi: 10.1016/0360-3016(88)90312-4. [DOI] [PubMed] [Google Scholar]
- Höckel M., Knoop C., Schlenger K., Vorndran B., Baussmann E., Mitze M., Knapstein P. G., Vaupel P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993 Jan;26(1):45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- Kallinowski F., Zander R., Hoeckel M., Vaupel P. Tumor tissue oxygenation as evaluated by computerized-pO2-histography. Int J Radiat Oncol Biol Phys. 1990 Oct;19(4):953–961. doi: 10.1016/0360-3016(90)90018-f. [DOI] [PubMed] [Google Scholar]
- Kjellen E., Joiner M. C., Collier J. M., Johns H., Rojas A. A therapeutic benefit from combining normobaric carbogen or oxygen with nicotinamide in fractionated X-ray treatments. Radiother Oncol. 1991 Oct;22(2):81–91. doi: 10.1016/0167-8140(91)90002-x. [DOI] [PubMed] [Google Scholar]
- Reinhold H. S., Blachiwiecz B., Blok A. Oxygenation and reoxygenation in 'sandwich' tumours. Bibl Anat. 1977;(15 Pt 1):270–272. [PubMed] [Google Scholar]
- Rubin P., Hanley J., Keys H. M., Marcial V., Brady L. Carbogen breathing during radiation therapy-the Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys. 1979 Nov-Dec;5(11-12):1963–1970. doi: 10.1016/0360-3016(79)90946-5. [DOI] [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter M. J., Chaplin D. J., Durand R. E., Olive P. L. The use of fluorescent probes to identify regions of transient perfusion in murine tumors. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):931–934. doi: 10.1016/0360-3016(89)90889-4. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Schlenger K., Knoop C., Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991 Jun 15;51(12):3316–3322. [PubMed] [Google Scholar]
- Zackrisson B., Franzén L., Henriksson R., Littbrand B., Stratford M., Dennis M., Rojas A. M., Denekamp J. Acute effects of accelerated radiotherapy in combination with carbogen breathing and nicotinamide (ARCON). Acta Oncol. 1994;33(4):377–381. doi: 10.3109/02841869409098432. [DOI] [PubMed] [Google Scholar]