Abstract
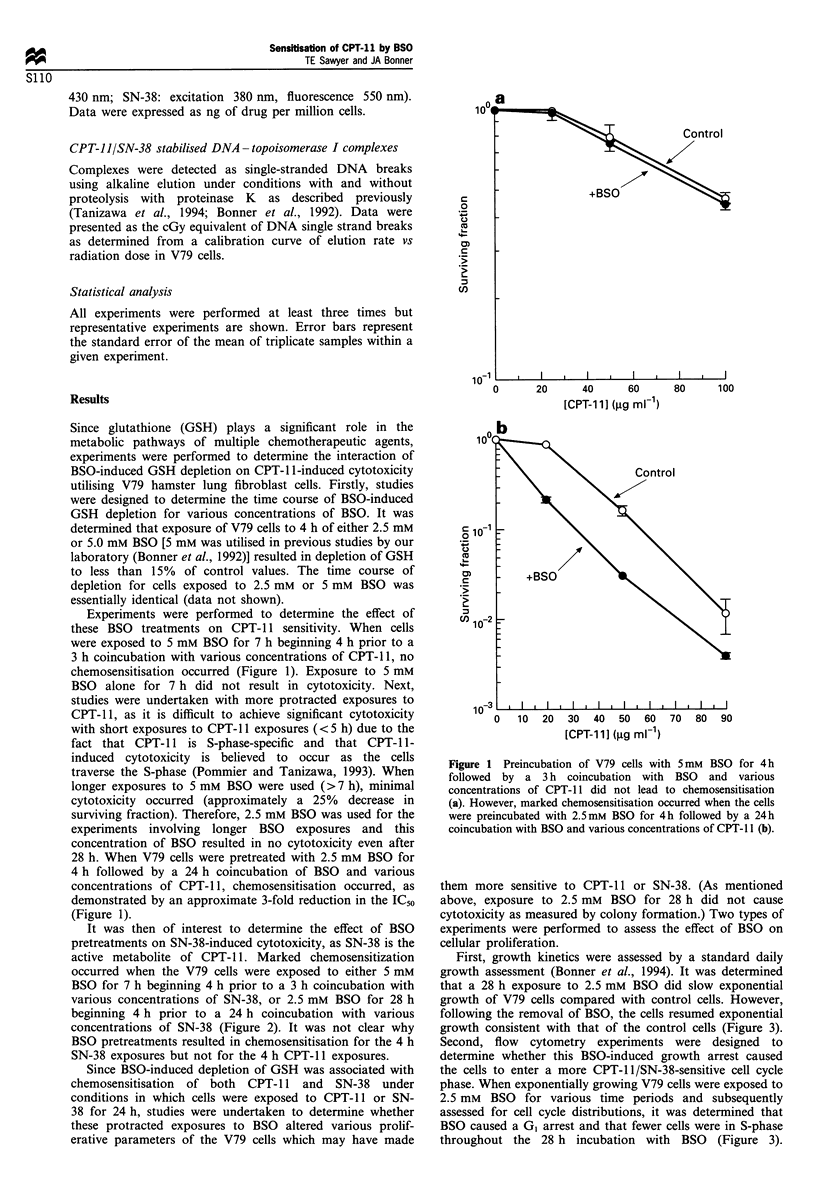
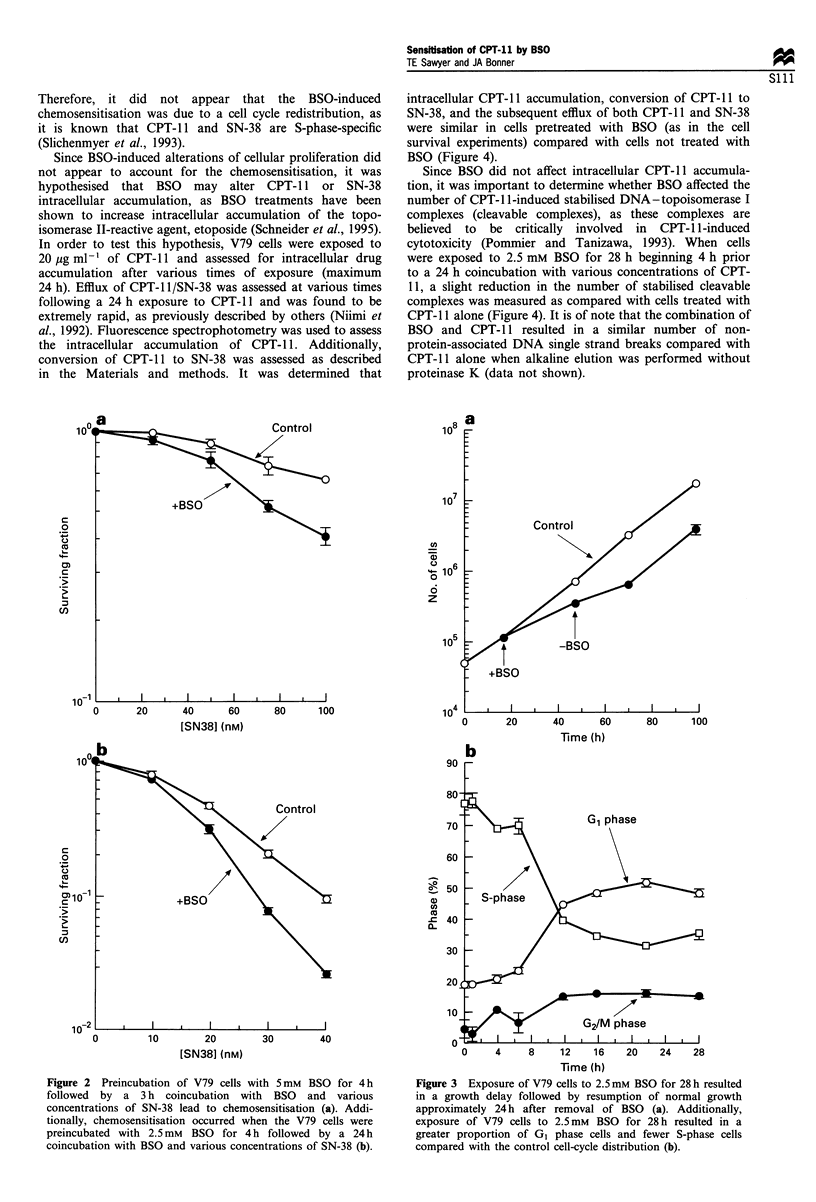
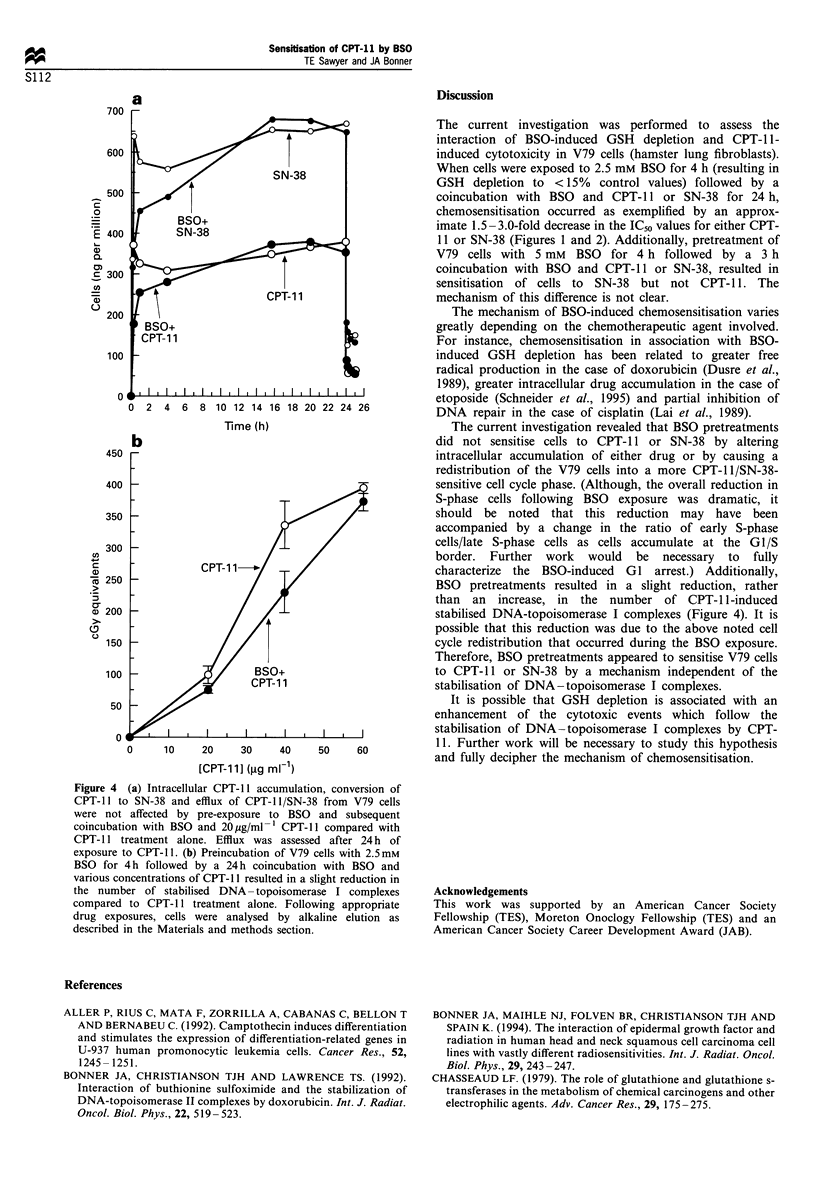
Buthionine sulphoximide (BSO)-induced depletion of glutathione (GSH) was found to be associated with an increased sensitivity to CPT-11 (topoisomerase I-reactive agent) in V79 hamster lung fibroblast cells. When V79 cells were exposed to 2.5 mM BSO for 28 h beginning 4 h prior to a 24 h coincubation with CPT-11, cytotoxicity was increased compared with CPT-11 alone. It was determined that BSO resulted in a G1 cell cycle arrest and a decrease in the percentage of cells in S-phase. Since CPT-11 is known to be S-phase-specific, this BSO-induced cell cycle redistribution did not appear to account for the chemosensitisation of CPT-11. Additionally, BSO did not alter intracellular accumulation of CPT-11, conversion of CPT-11 to its active metabolite SN-38, or efflux of either CPT-11 or SN-38 from the cell. Finally, BSO resulted in a slight reduction, rather than an increase, in the number of stabilised DNA-topoisomerase I complexes induced by CPT-11. Therefore, these results suggest that BSO-induced sensitisation of V79 cells to the cytotoxic effects of CPT-11 occurs by a mechanism independent of the stabilisation of DNA-topoisomerase I complexes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aller P., Rius C., Mata F., Zorrilla A., Cabañas C., Bellón T., Bernabeu C. Camptothecin induces differentiation and stimulates the expression of differentiation-related genes in U-937 human promonocytic leukemia cells. Cancer Res. 1992 Mar 1;52(5):1245–1251. [PubMed] [Google Scholar]
- Bonner J. A., Christianson T. J., Lawrence T. S. Interaction of buthionine sulfoximine and the stabilization of DNA-topoisomerase II complexes by doxorubicin. Int J Radiat Oncol Biol Phys. 1992;22(3):519–523. doi: 10.1016/0360-3016(92)90866-g. [DOI] [PubMed] [Google Scholar]
- Bonner J. A., Maihle N. J., Folven B. R., Christianson T. J., Spain K. The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivities. Int J Radiat Oncol Biol Phys. 1994 May 15;29(2):243–247. doi: 10.1016/0360-3016(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Steinkamp J. A. Rapid, one step staining procedures for analysis of cellular DNA and protein by single and dual laser flow cytometry. Cytometry. 1982 Sep;3(2):84–90. doi: 10.1002/cyto.990030204. [DOI] [PubMed] [Google Scholar]
- Dusre L., Mimnaugh E. G., Myers C. E., Sinha B. K. Potentiation of doxorubicin cytotoxicity by buthionine sulfoximine in multidrug-resistant human breast tumor cells. Cancer Res. 1989 Feb 1;49(3):511–515. [PubMed] [Google Scholar]
- Lai G. M., Ozols R. F., Young R. C., Hamilton T. C. Effect of glutathione on DNA repair in cisplatin-resistant human ovarian cancer cell lines. J Natl Cancer Inst. 1989 Apr 5;81(7):535–539. doi: 10.1093/jnci/81.7.535. [DOI] [PubMed] [Google Scholar]
- Niimi S., Nakagawa K., Sugimoto Y., Nishio K., Fujiwara Y., Yokoyama S., Terashima Y., Saijo N. Mechanism of cross-resistance to a camptothecin analogue (CPT-11) in a human ovarian cancer cell line selected by cisplatin. Cancer Res. 1992 Jan 15;52(2):328–333. [PubMed] [Google Scholar]
- Rice G. C., Bump E. A., Shrieve D. C., Lee W., Kovacs M. Quantitative analysis of cellular glutathione by flow cytometry utilizing monochlorobimane: some applications to radiation and drug resistance in vitro and in vivo. Cancer Res. 1986 Dec;46(12 Pt 1):6105–6110. [PubMed] [Google Scholar]
- Schaak J., Schedl P., Shenk T. Transcription of adenovirus and HeLa cell genes in the presence of drugs that inhibit topoisomerase I and II function. Nucleic Acids Res. 1990 Mar 25;18(6):1499–1508. doi: 10.1093/nar/18.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Yamazaki H., Sinha B. K., Cowan K. H. Buthionine sulphoximine-mediated sensitisation of etoposide-resistant human breast cancer MCF7 cells overexpressing the multidrug resistance-associated protein involves increased drug accumulation. Br J Cancer. 1995 Apr;71(4):738–743. doi: 10.1038/bjc.1995.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slichenmyer W. J., Rowinsky E. K., Donehower R. C., Kaufmann S. H. The current status of camptothecin analogues as antitumor agents. J Natl Cancer Inst. 1993 Feb 17;85(4):271–291. doi: 10.1093/jnci/85.4.271. [DOI] [PubMed] [Google Scholar]
- Tanizawa A., Fujimori A., Fujimori Y., Pommier Y. Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J Natl Cancer Inst. 1994 Jun 1;86(11):836–842. doi: 10.1093/jnci/86.11.836. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang J. C., Liu L. F. Involvement of DNA topoisomerase I in transcription of human ribosomal RNA genes. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1060–1064. doi: 10.1073/pnas.85.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]


