Abstract
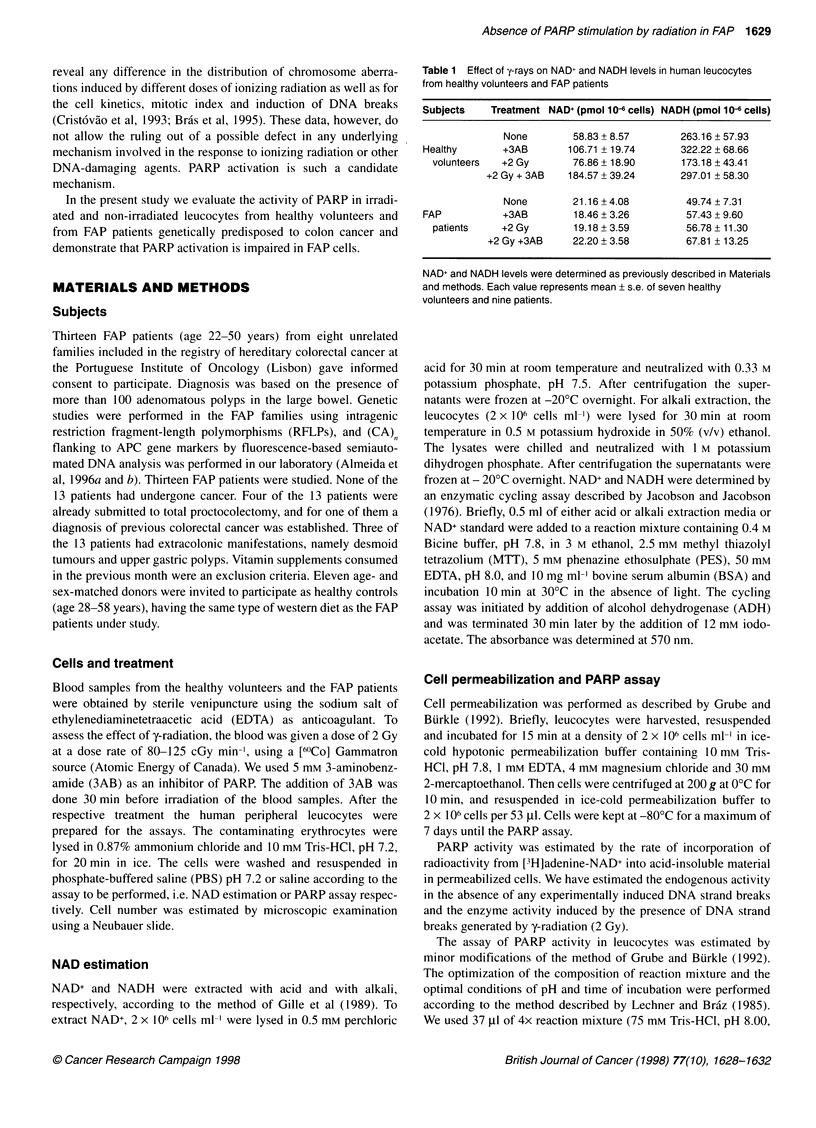
Poly(ADP-ribose)polymerase (PARP) has been implicated in DNA repair mechanisms and the associated activity shown to markedly increase after DNA damage in carcinogen-treated cells. A defective DNA repair has been associated to the aetiology of human cancers. In order to assess the potential role of this enzyme in cellular response to DNA damage by gamma-radiation, we studied the activity of PARP in patients with familial adenomatous polyposis (FAP). We compared poly(ADP-ribose)polymerase activity by the rate of incorporation of radioactivity from [3H]adenine-NAD+ into acid-insoluble material in permeabilized leucocytes from FAP patients and healthy volunteers. Concomitantly, the intracellular levels of NAD+--the substrate for the PARP--and the reduced counterpart NADH were determined using an enzymatic cycling assay 30 min after [60Co] gamma-ray cells irradiation. Our results demonstrate that a marked stimulation of PARP activity is produced upon radiation of the cells from healthy subjects but not in the FAP leucocytes, which concomitantly show a marked decrease in total NAD-/NADH content. Our observations point to a role of PARP in the repair of the gamma-radiation-induced DNA lesions through a mechanism that is impaired in the cells from FAP patients genetically predisposed to colon cancer. The differences observed in PARP activation by gamma-radiation in patients and healthy individuals could reflect the importance of PARP activity dependent on treatment with gamma-rays. The absence of this response in FAP patients would seem to suggest a possible defect in the role of PARP in radiation-induced DNA repair in this cancer-prone disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida R., Fidalgo P., Ramalho E., Brás A., Leitão N., Mira C., Rueff J., Monteiro C. Presymptomatic diagnosis in Portuguese FAP families using intragenic RFLPs and (CA)n flanking markers by fluorescence based semiautomated DNA analysis. J Med Genet. 1996 Mar;33(3):244–247. doi: 10.1136/jmg.33.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida R., Morton N., Fidalgo P., Leitão N., Mira C., Rueff J., Monteiro C. APC intragenic haplotypes in familial adenomatous polyposis. Clin Genet. 1996 Dec;50(6):483–485. doi: 10.1111/j.1399-0004.1996.tb02717.x. [DOI] [PubMed] [Google Scholar]
- Benjamin R. C., Gill D. M. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980 Nov 10;255(21):10502–10508. [PubMed] [Google Scholar]
- Birnboim H. C. DNA strand breaks in human leukocytes induced by superoxide anion, hydrogen peroxide and tumor promoters are repaired slowly compared to breaks induced by ionizing radiation. Carcinogenesis. 1986 Sep;7(9):1511–1517. doi: 10.1093/carcin/7.9.1511. [DOI] [PubMed] [Google Scholar]
- Bièche I., de Murcia G., Lidereau R. Poly(ADP-ribose) polymerase gene expression status and genomic instability in human breast cancer. Clin Cancer Res. 1996 Jul;2(7):1163–1167. [PubMed] [Google Scholar]
- Bràz J., Lechner M. C. ADP-ribosylation of nuclear proteins is increased by phenobarbital. Identification of the ADP-ribosylated histone fractions in rat liver nuclei. FEBS Lett. 1986 Apr 21;199(2):164–168. doi: 10.1016/0014-5793(86)80472-0. [DOI] [PubMed] [Google Scholar]
- Brás A., Cristóvão L., Coelho C., Hilali A., Dutrillaux B., Léonard A., Rueff J. Normal genetic response to gamma irradiation in familial adenomatous polyposis. Eur J Cancer. 1995;31A(9):1506–1510. doi: 10.1016/0959-8049(95)00247-g. [DOI] [PubMed] [Google Scholar]
- Bürkle A., Müller M., Wolf I., Küpper J. H. Poly(ADP-ribose) polymerase activity in intact or permeabilized leukocytes from mammalian species of different longevity. Mol Cell Biochem. 1994 Sep;138(1-2):85–90. [PubMed] [Google Scholar]
- Cleaver J. E. It was a very good year for DNA repair. Cell. 1994 Jan 14;76(1):1–4. doi: 10.1016/0092-8674(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Cristóvão L., Rueff J. Effect of a poly(ADP-ribose) polymerase inhibitor on DNA breakage and cytotoxicity induced by hydrogen peroxide and gamma-radiation. Teratog Carcinog Mutagen. 1996;16(4):219–227. doi: 10.1002/(SICI)1520-6866(1996)16:4<219::AID-TCM3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Flick K., Schneider R., Auer B., Hirsch-Kauffmann M., Schweiger M. No abnormalities in the NAD+ ADP-ribosyltransferase (polymerizing) gene of transformed cells from a Fanconi's anemia patient. Hum Genet. 1992 Aug;89(6):690–691. doi: 10.1007/BF00221967. [DOI] [PubMed] [Google Scholar]
- Gille J. J., van Berkel C. G., Mullaart E., Vijg J., Joenje H. Effects of lethal exposure to hyperoxia and to hydrogen peroxide on NAD(H) and ATP pools in Chinese hamster ovary cells. Mutat Res. 1989 Sep;214(1):89–96. doi: 10.1016/0027-5107(89)90201-7. [DOI] [PubMed] [Google Scholar]
- Gradwohl G., Ménissier de Murcia J. M., Molinete M., Simonin F., Koken M., Hoeijmakers J. H., de Murcia G. The second zinc-finger domain of poly(ADP-ribose) polymerase determines specificity for single-stranded breaks in DNA. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2990–2994. doi: 10.1073/pnas.87.8.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin S. DNA damage, DNA repair and disease. Curr Biol. 1996 May 1;6(5):497–499. doi: 10.1016/s0960-9822(02)00525-0. [DOI] [PubMed] [Google Scholar]
- Grube K., Bürkle A. Poly(ADP-ribose) polymerase activity in mononuclear leukocytes of 13 mammalian species correlates with species-specific life span. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11759–11763. doi: 10.1073/pnas.89.24.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller B., Wang Z. Q., Wagner E. F., Radons J., Bürkle A., Fehsel K., Burkart V., Kolb H. Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J Biol Chem. 1995 May 12;270(19):11176–11180. doi: 10.1074/jbc.270.19.11176. [DOI] [PubMed] [Google Scholar]
- Ikejima M., Noguchi S., Yamashita R., Ogura T., Sugimura T., Gill D. M., Miwa M. The zinc fingers of human poly(ADP-ribose) polymerase are differentially required for the recognition of DNA breaks and nicks and the consequent enzyme activation. Other structures recognize intact DNA. J Biol Chem. 1990 Dec 15;265(35):21907–21913. [PubMed] [Google Scholar]
- Jacobson E. L., Jacobson M. K. Pyridine nucleotide levels as a function of growth in normal and transformed 3T3 cells. Arch Biochem Biophys. 1976 Aug;175(2):627–634. doi: 10.1016/0003-9861(76)90553-1. [DOI] [PubMed] [Google Scholar]
- Kolodner R. D. Mismatch repair: mechanisms and relationship to cancer susceptibility. Trends Biochem Sci. 1995 Oct;20(10):397–401. doi: 10.1016/s0968-0004(00)89087-8. [DOI] [PubMed] [Google Scholar]
- Lechner M. C., Bráz J. Nuclear ADP-ribosyl transferase activity correlates with induction of P-450 monooxygenases by phenobarbital in rat liver microsomes. Eur J Biochem. 1985 Sep 16;151(3):621–624. doi: 10.1111/j.1432-1033.1985.tb09149.x. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Satoh M. S., Dianov G. Enzymes acting at strand interruptions in DNA. Philos Trans R Soc Lond B Biol Sci. 1995 Jan 30;347(1319):57–62. doi: 10.1098/rstb.1995.0009. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Satoh M. S., Poirier G. G., Klungland A. Post-translational modification of poly(ADP-ribose) polymerase induced by DNA strand breaks. Trends Biochem Sci. 1995 Oct;20(10):405–411. doi: 10.1016/s0968-0004(00)89089-1. [DOI] [PubMed] [Google Scholar]
- Markowitz M. M., Rozen P., Pero R. W., Tobi M., Miller D. G. Hydrogen peroxide induced adenosine diphosphate ribosyl transferase (ADPRT) response in patients with inflammatory bowel disease. Gut. 1988 Dec;29(12):1680–1686. doi: 10.1136/gut.29.12.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinete M., Vermeulen W., Bürkle A., Ménissier-de Murcia J., Küpper J. H., Hoeijmakers J. H., de Murcia G. Overproduction of the poly(ADP-ribose) polymerase DNA-binding domain blocks alkylation-induced DNA repair synthesis in mammalian cells. EMBO J. 1993 May;12(5):2109–2117. doi: 10.1002/j.1460-2075.1993.tb05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane J. P. Role of induced genetic instability in the mutagenic effects of chemicals and radiation. Mutat Res. 1996 Jan;367(1):11–23. [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Honjo T., Hayaishi O. Enzymic adenosine diphosphate ribosylation of histone and poly adenosine diphosphate ribose synthesis in rat liver nuclei. J Biol Chem. 1968 Jul 10;243(13):3765–3767. [PubMed] [Google Scholar]
- Panzeter P. L., Althaus F. R. DNA strand break-mediated partitioning of poly(ADP-ribose) polymerase function. Biochemistry. 1994 Aug 16;33(32):9600–9605. doi: 10.1021/bi00198a028. [DOI] [PubMed] [Google Scholar]
- Sancar A. DNA repair in humans. Annu Rev Genet. 1995;29:69–105. doi: 10.1146/annurev.ge.29.120195.000441. [DOI] [PubMed] [Google Scholar]
- Satoh M. S., Lindahl T. Enzymatic repair of oxidative DNA damage. Cancer Res. 1994 Apr 1;54(7 Suppl):1899s–1901s. [PubMed] [Google Scholar]
- Satoh M. S., Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature. 1992 Mar 26;356(6367):356–358. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- Satoh M. S., Poirier G. G., Lindahl T. Dual function for poly(ADP-ribose) synthesis in response to DNA strand breakage. Biochemistry. 1994 Jun 14;33(23):7099–7106. doi: 10.1021/bi00189a012. [DOI] [PubMed] [Google Scholar]
- Satoh M. S., Poirier G. G., Lindahl T. NAD(+)-dependent repair of damaged DNA by human cell extracts. J Biol Chem. 1993 Mar 15;268(8):5480–5487. [PubMed] [Google Scholar]
- Schweiger M., Auer B., Burtscher H. J., Hirsch-Kauffmann M., Klocker H., Schneider R. The Fritz-Lipmann lecture. DNA repair in human cells. Biochemistry of the hereditary diseases Fanconi's anaemia and Cockayne syndrome. Eur J Biochem. 1987 Jun 1;165(2):235–242. doi: 10.1111/j.1432-1033.1987.tb11433.x. [DOI] [PubMed] [Google Scholar]
- Skidmore C. J., Davies M. I., Goodwin P. M., Halldorsson H., Lewis P. J., Shall S., Zia'ee A. A. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem. 1979 Nov 1;101(1):135–142. doi: 10.1111/j.1432-1033.1979.tb04225.x. [DOI] [PubMed] [Google Scholar]


