Abstract
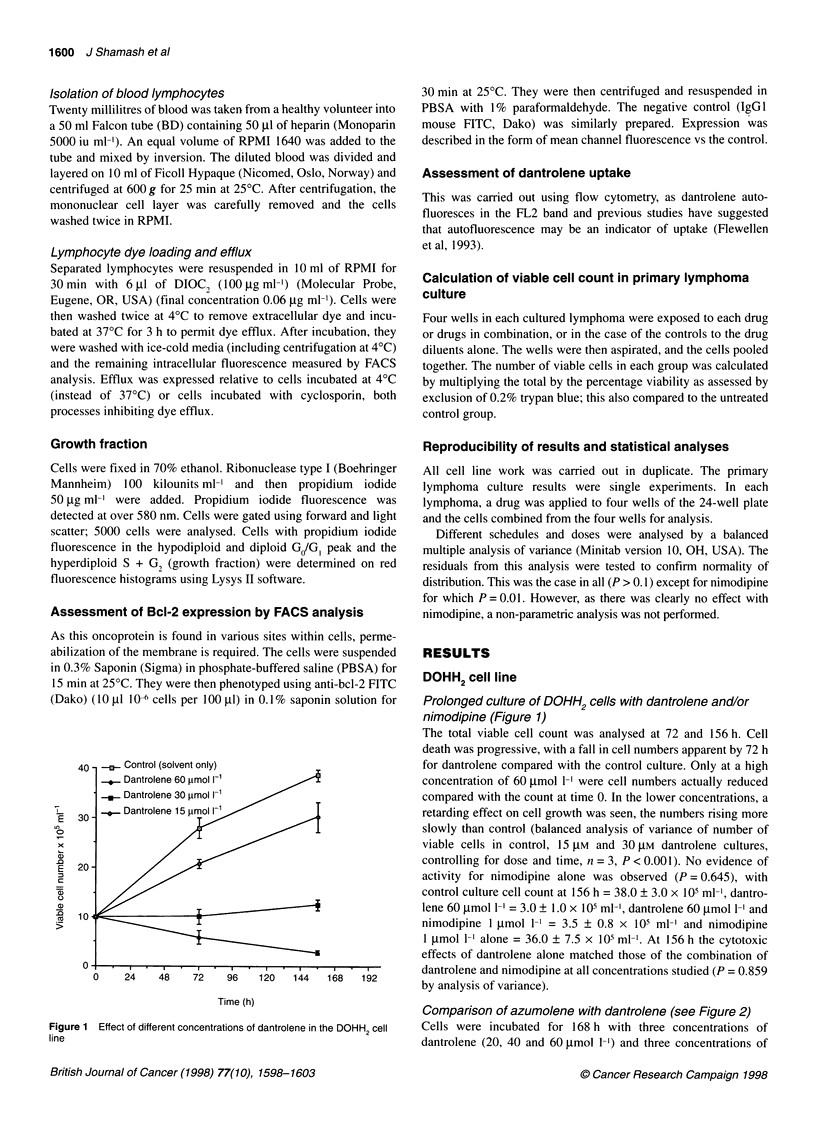
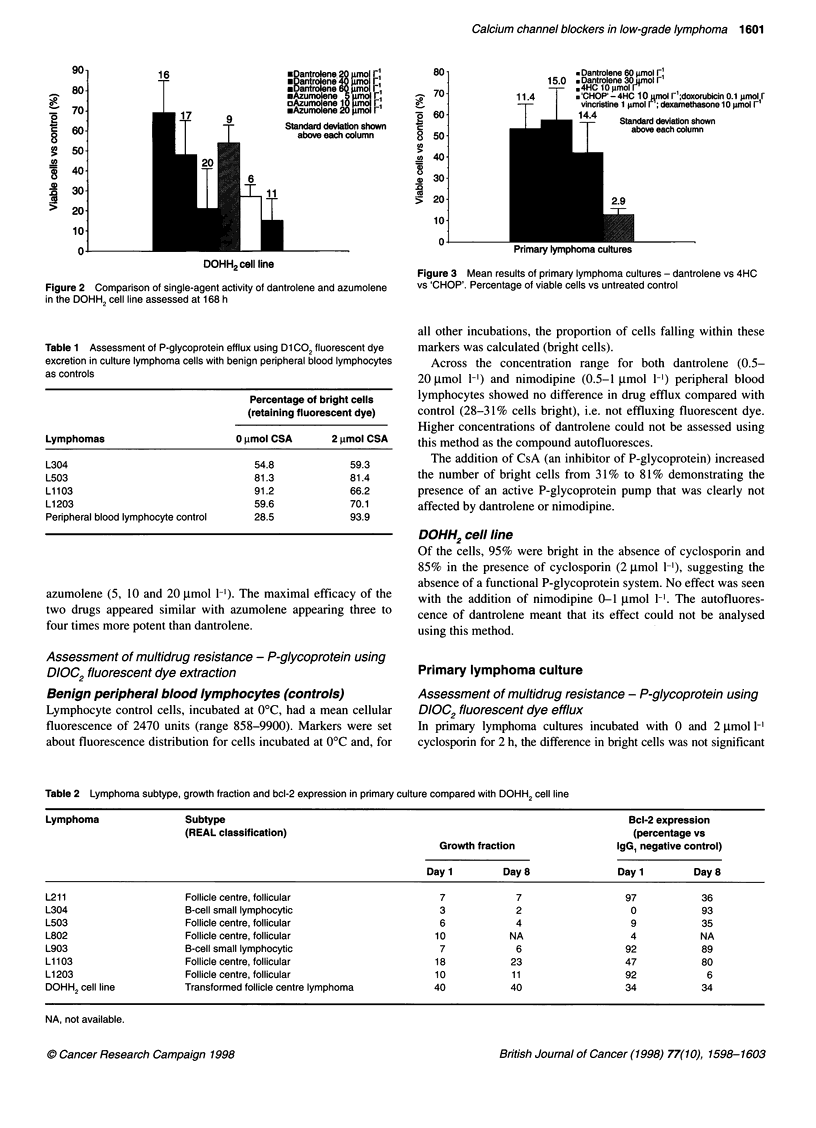
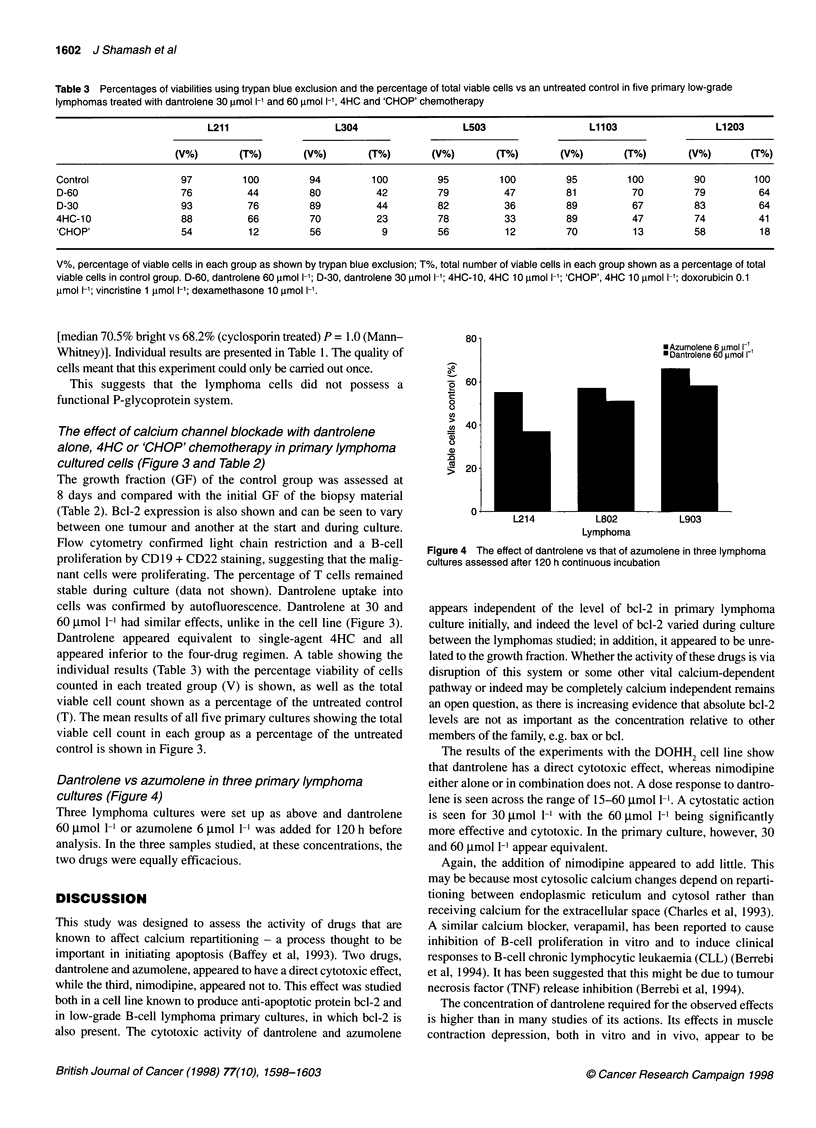
The flux of calcium forms an important intracellular messenger system. The bcl-2 oncoprotein is thought to make cells resistant to a variety of insults, including cytotoxic drugs, by the suppression of apoptosis, which appears to involve the repartitioning of intracellular calcium. Three drugs that affect calcium pathways and may influence this repartitioning, i.e. dantrolene, azumolene (a water-soluble dantrolene analogue) and nimodipine, were studied in cell culture, using both a transformed follicle centre lymphoma cell line and primary culture of lymphoma cells in vitro in a manner that resulted in a growth pattern closely resembling that of the malignancy in vivo. Dantrolene and azumolene were potent inducers of cell death in both systems reducing the viable cell count by 70-90% in comparison with normal controls. Nimodipine, in comparison, appeared to have no significant effect. These results obtained in an in vitro setting suggest that further evaluation of dantrolene and azumolene for the treatment of low-grade non-Hodgkin's lymphoma is warranted.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baffy G., Miyashita T., Williamson J. R., Reed J. C. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993 Mar 25;268(9):6511–6519. [PubMed] [Google Scholar]
- Berrebi A., Shtalrid M., Klepfish A., Bassous L., Kushnir M., Shulman L., Vorst E., Hahn T. Verapamil inhibits B-cell proliferation and tumor necrosis factor release and induces a clinical response in B-cell chronic lymphocytic leukemia. Leukemia. 1994 Dec;8(12):2214–2216. [PubMed] [Google Scholar]
- Berridge M. J. Cell signalling. A tale of two messengers. Nature. 1993 Sep 30;365(6445):388–389. doi: 10.1038/365388a0. [DOI] [PubMed] [Google Scholar]
- Blank J. W., Boggs S. D. Successful treatment of an episode of malignant hyperthermia using a large dose of dantrolene. J Clin Anesth. 1993 Jan-Feb;5(1):69–72. doi: 10.1016/0952-8180(93)90092-s. [DOI] [PubMed] [Google Scholar]
- Charles A. C., Dirksen E. R., Merrill J. E., Sanderson M. J. Mechanisms of intercellular calcium signaling in glial cells studied with dantrolene and thapsigargin. Glia. 1993 Feb;7(2):134–145. doi: 10.1002/glia.440070203. [DOI] [PubMed] [Google Scholar]
- Chaudhary P. M., Mechetner E. B., Roninson I. B. Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes. Blood. 1992 Dec 1;80(11):2735–2739. [PubMed] [Google Scholar]
- De Erausquin G., Brooker G., Costa E., Wojcik W. J. Stimulation of high affinity gamma-aminobutyric acidB receptors potentiates the depolarization-induced increase of intraneuronal ionized calcium content in cerebellar granule neurons. Mol Pharmacol. 1992 Sep;42(3):407–414. [PubMed] [Google Scholar]
- Dershwitz M., Sréter F. A. Azumolene reverses episodes of malignant hyperthermia in susceptible swine. Anesth Analg. 1990 Mar;70(3):253–255. doi: 10.1213/00000539-199003000-00004. [DOI] [PubMed] [Google Scholar]
- Ellis K. O., Butterfield J. L., Wessels F. L., Carpenter J. F. A comparison of skeletal, cardiac, and smooth muscle actions of dantrolene sodium--a skeletal muscle relaxant. Arch Int Pharmacodyn Ther. 1976 Nov;224(1):118–132. [PubMed] [Google Scholar]
- Flewellen E. H., Nelson T. E., Jones W. P., Arens J. F., Wagner D. L. Dantrolene dose response in awake man: implications for management of malignant hyperthermia. Anesthesiology. 1983 Oct;59(4):275–280. doi: 10.1097/00000542-198310000-00002. [DOI] [PubMed] [Google Scholar]
- Galione A. Cyclic ADP-ribose: a new way to control calcium. Science. 1993 Jan 15;259(5093):325–326. doi: 10.1126/science.8380506. [DOI] [PubMed] [Google Scholar]
- Garrido J. J., Arahuetes R. M., Hernanz A., De la Fuente M. Modulation by neurotensin and neuromedin N of adherence and chemotaxis capacity of murine lymphocytes. Regul Pept. 1992 Sep 3;41(1):27–37. doi: 10.1016/0167-0115(92)90511-r. [DOI] [PubMed] [Google Scholar]
- Harris N. L., Jaffe E. S., Stein H., Banks P. M., Chan J. K., Cleary M. L., Delsol G., De Wolf-Peeters C., Falini B., Gatter K. C. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994 Sep 1;84(5):1361–1392. [PubMed] [Google Scholar]
- Koike M., Kashiwagura T., Takeguchi N. Gluconeogenesis stimulated by extracellular ATP is triggered by the initial increase in the intracellular Ca2+ concentration of the periphery of hepatocytes. Biochem J. 1992 Apr 1;283(Pt 1):265–272. doi: 10.1042/bj2830265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M., Dubyak G., Chen L., Nuñez G., Miesfeld R. L., Distelhorst C. W. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie G. C., Part N. J. The effect of EU4093 (azumolene sodium) on the contraction of intrafusal muscle in the soleus muscle of the anaesthetized rat. Br J Pharmacol. 1989 Aug;97(4):1151–1156. doi: 10.1111/j.1476-5381.1989.tb12573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister T. A., Cullen M. H., Beard M. E., Brearley R. L., Whitehouse J. M., Wrigley P. F., Stansfeld A. G., Sutcliffe S. B., Malpas J. S., Crowther D. Comparison of combined and single-agent chemotherapy in non-Hodgkin's lymphoma of favourable histological type. Br Med J. 1978 Mar 4;1(6112):533–537. doi: 10.1136/bmj.1.6112.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto S., Carrera C. J., Kubota M., Wasson D. B., Carson D. A. Mechanism of deoxyadenosine and 2-chlorodeoxyadenosine toxicity to nondividing human lymphocytes. J Clin Invest. 1985 Feb;75(2):377–383. doi: 10.1172/JCI111710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P., Gerasimenko O., Petersen O. H. Cyclic ADP-ribose regulation of ryanodine receptors involved in agonist evoked cytosolic Ca2+ oscillations in pancreatic acinar cells. EMBO J. 1994 May 1;13(9):2038–2043. doi: 10.1002/j.1460-2075.1994.tb06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q., Katz A. M., Kim D. H. Effects of azumolene on doxorubicin-induced Ca2+ release from skeletal and cardiac muscle sarcoplasmic reticulum. Biochim Biophys Acta. 1991 Aug 13;1094(1):27–34. doi: 10.1016/0167-4889(91)90022-p. [DOI] [PubMed] [Google Scholar]


