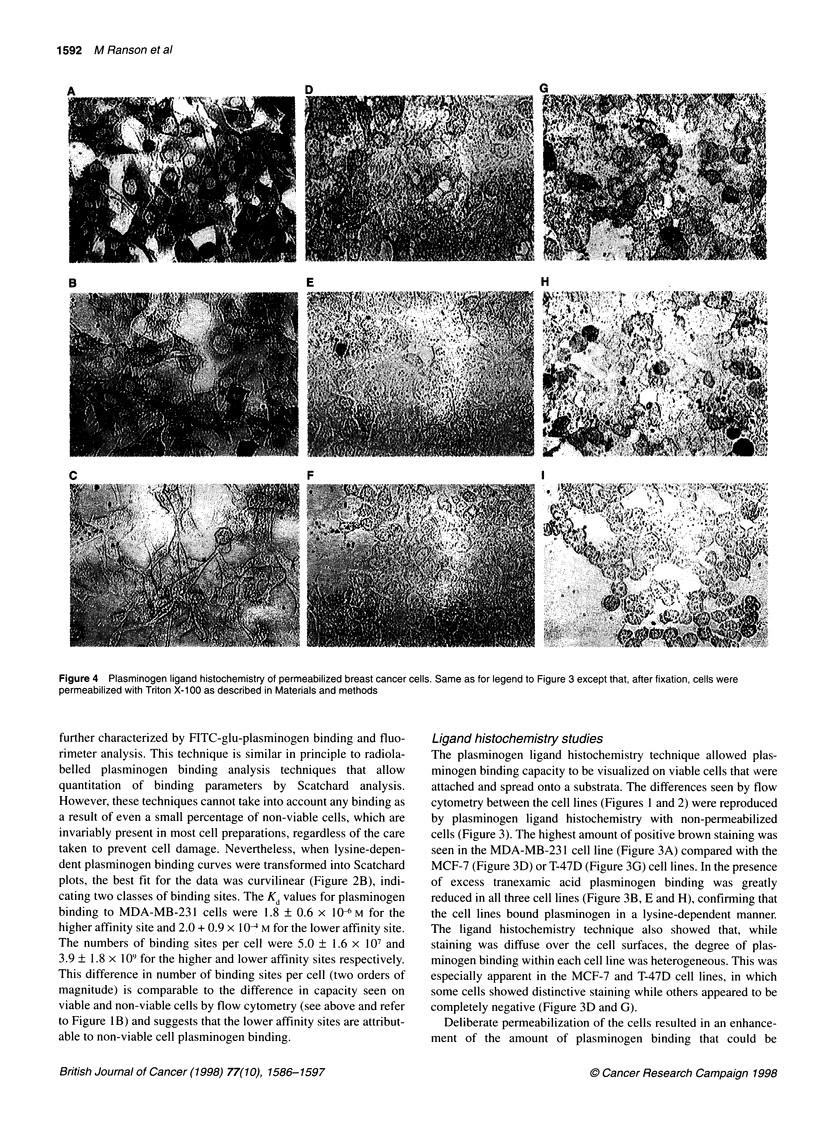
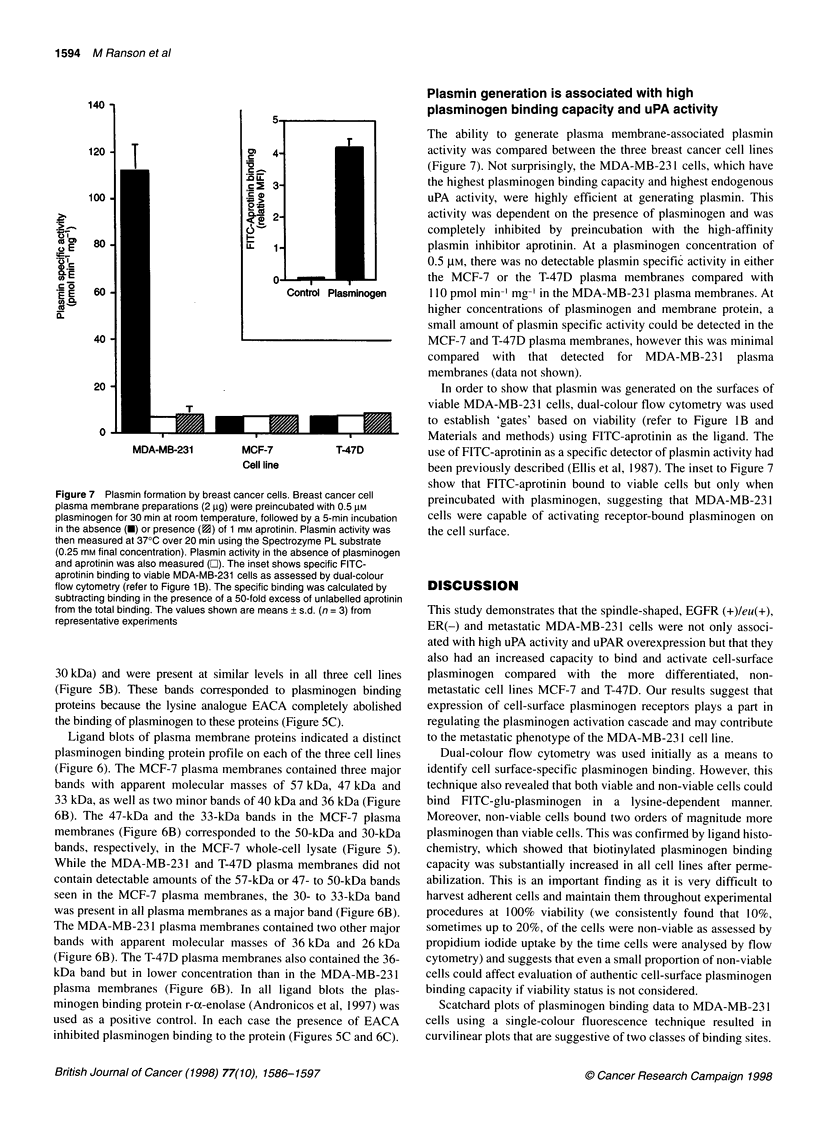
Abstract
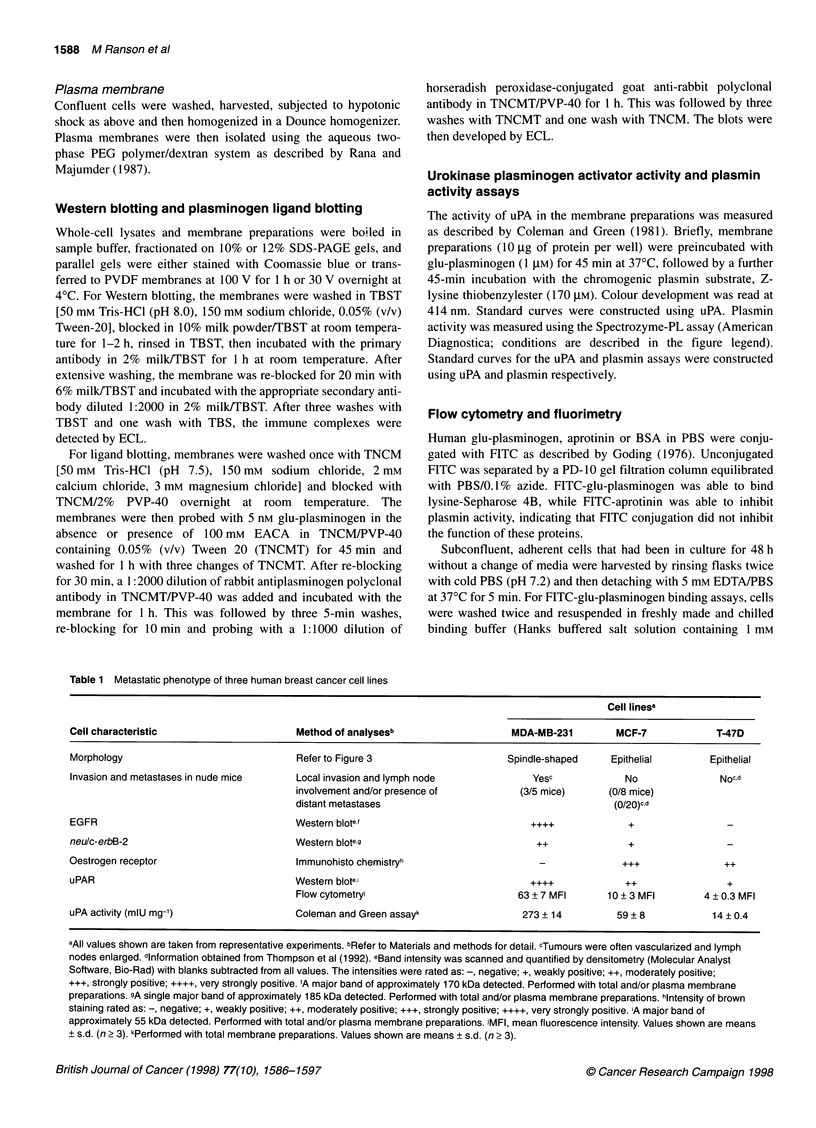
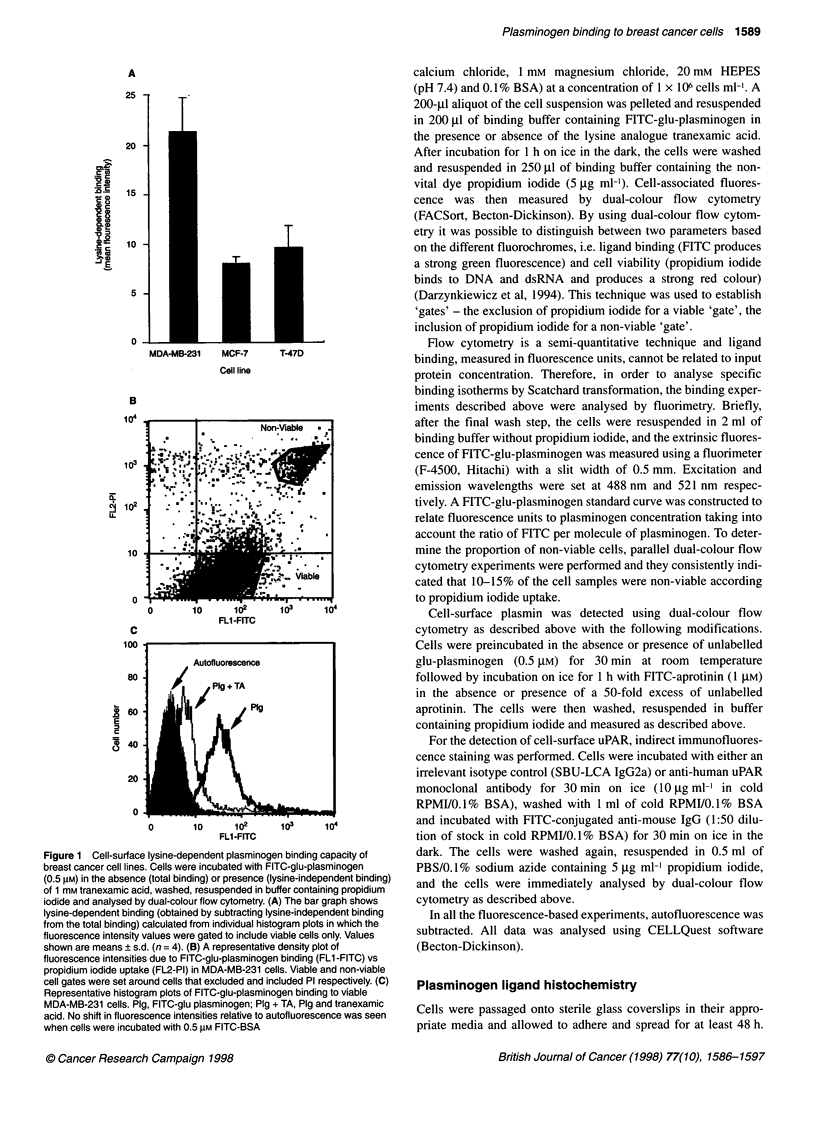
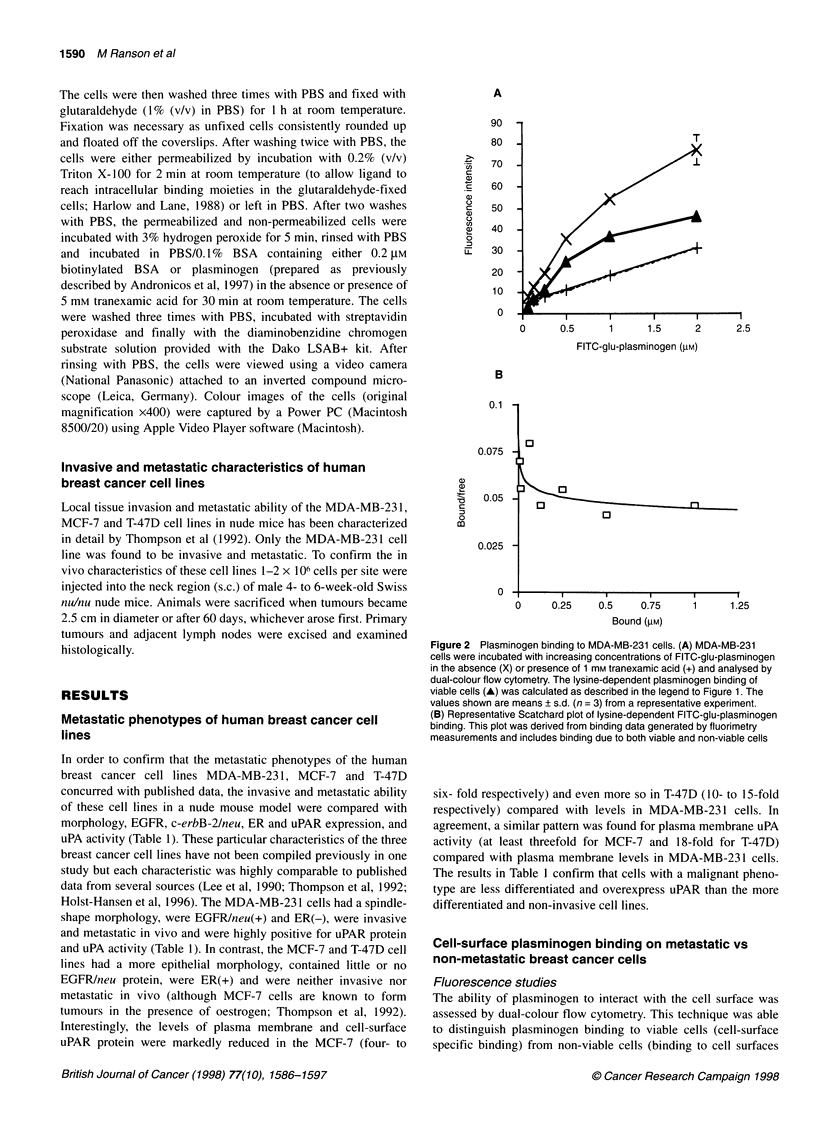
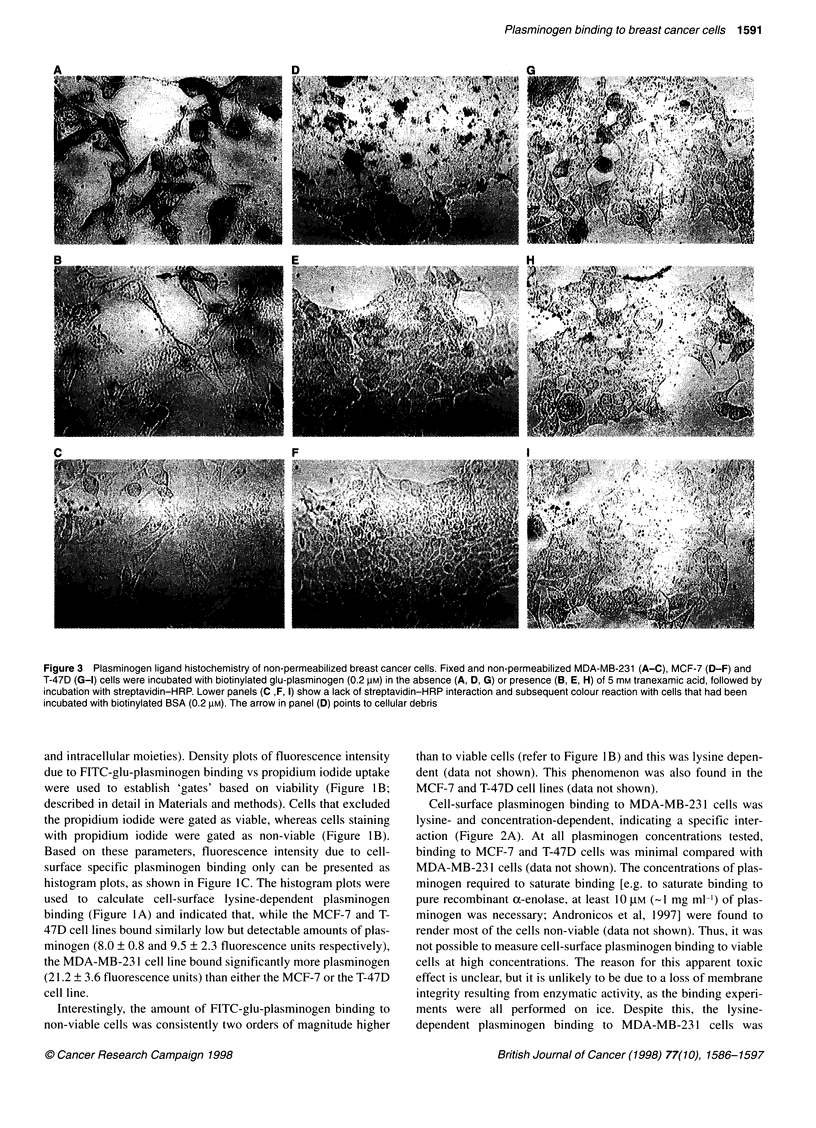
Overexpression of urokinase-type plasminogen activator and its receptor correlates with metastatic capacity in breast cancer. In this study we show that the urokinase/urokinase receptor-overexpressing, metastatic human breast cancer cell line MDA-MB-231 (1) bound significantly more cell-surface plasminogen in a lysine-dependent manner and (2) was capable of generating large amounts of plasmin compared with the non-metastatic cell lines MCF-7 and T-47D. In addition, distinct plasminogen binding proteins were detected in the plasma membranes of the cell lines, suggesting heterogeneity of binding proteins. Plasminogen binding was analysed using a combination of dual-colour fluorescence flow cytometry and ligand histochemistry (for comparative and cellular localization of ligand binding), and fluorimetry (for Scatchard analysis). Apart from revealing the greater plasminogen binding capacity of MDA-MB-231 cells, flow cytometry and histochemistry also revealed that, in all three cell lines, non-viable or permeabilized cells bound significantly more plasminogen in a lysine-dependent manner than viable or non-permeabilized cells. Viable MDA-MB-231 cells bound plasminogen with moderate affinity and high capacity (Kd = 1.8 microM, receptor sites per cell 5.0 x 10(7). Our results indicate that differences in cell surface-specific plasminogen binding capacity between cell lines may not be detectable with binding techniques that cannot distinguish between viable and non-viable cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andronicos N. M., Ranson M., Bognacki J., Baker M. S. The human ENO1 gene product (recombinant human alpha-enolase) displays characteristics required for a plasminogen binding protein. Biochim Biophys Acta. 1997 Jan 4;1337(1):27–39. doi: 10.1016/s0167-4838(96)00146-x. [DOI] [PubMed] [Google Scholar]
- Azzam H. S., Arand G., Lippman M. E., Thompson E. W. Association of MMP-2 activation potential with metastatic progression in human breast cancer cell lines independent of MMP-2 production. J Natl Cancer Inst. 1993 Nov 3;85(21):1758–1764. doi: 10.1093/jnci/85.21.1758. [DOI] [PubMed] [Google Scholar]
- Baker M. S., Bleakley P., Woodrow G. C., Doe W. F. Inhibition of cancer cell urokinase plasminogen activator by its specific inhibitor PAI-2 and subsequent effects on extracellular matrix degradation. Cancer Res. 1990 Aug 1;50(15):4676–4684. [PubMed] [Google Scholar]
- Burtin P., Zhang S., Schauffler J., Komano O., Sastre X., Mathieu M. C. Visualization of the plasmin receptor on sections of human mammary carcinoma cells. Int J Cancer. 1993 Jan 2;53(1):17–21. doi: 10.1002/ijc.2910530105. [DOI] [PubMed] [Google Scholar]
- Busso N., Masur S. K., Lazega D., Waxman S., Ossowski L. Induction of cell migration by pro-urokinase binding to its receptor: possible mechanism for signal transduction in human epithelial cells. J Cell Biol. 1994 Jul;126(1):259–270. doi: 10.1083/jcb.126.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman G. M., Guevara C. A., Hajjar K. A. An endothelial cell receptor for plasminogen/tissue plasminogen activator (t-PA). II. Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J Biol Chem. 1994 Aug 19;269(33):21198–21203. [PubMed] [Google Scholar]
- Christensen L., Wiborg Simonsen A. C., Heegaard C. W., Moestrup S. K., Andersen J. A., Andreasen P. A. Immunohistochemical localization of urokinase-type plasminogen activator, type-1 plasminogen-activator inhibitor, urokinase receptor and alpha(2)-macroglobulin receptor in human breast carcinomas. Int J Cancer. 1996 May 16;66(4):441–452. doi: 10.1002/(SICI)1097-0215(19960516)66:4<441::AID-IJC6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Coleman P. L., Green G. D. A sensitive, coupled assay for plasminogen activator using a thiol ester substrate for plasmin. Ann N Y Acad Sci. 1981;370:617–626. doi: 10.1111/j.1749-6632.1981.tb29768.x. [DOI] [PubMed] [Google Scholar]
- Costantini V., Sidoni A., Deveglia R., Cazzato O. A., Bellezza G., Ferri I., Bucciarelli E., Nenci G. G. Combined overexpression of urokinase, urokinase receptor, and plasminogen activator inhibitor-1 is associated with breast cancer progression: an immunohistochemical comparison of normal, benign, and malignant breast tissues. Cancer. 1996 Mar 15;77(6):1079–1088. doi: 10.1002/(sici)1097-0142(19960315)77:6<1079::aid-cncr12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Culty M., Shizari M., Thompson E. W., Underhill C. B. Binding and degradation of hyaluronan by human breast cancer cell lines expressing different forms of CD44: correlation with invasive potential. J Cell Physiol. 1994 Aug;160(2):275–286. doi: 10.1002/jcp.1041600209. [DOI] [PubMed] [Google Scholar]
- Ellis V., Scully M. F., Kakkar V. V. Plasminogen activation by single-chain urokinase in functional isolation. A kinetic study. J Biol Chem. 1987 Nov 5;262(31):14998–15003. [PubMed] [Google Scholar]
- Foekens J. A., Buessecker F., Peters H. A., Krainick U., van Putten W. L., Look M. P., Klijn J. G., Kramer M. D. Plasminogen activator inhibitor-2: prognostic relevance in 1012 patients with primary breast cancer. Cancer Res. 1995 Apr 1;55(7):1423–1427. [PubMed] [Google Scholar]
- Ganz P. R., Dupuis D., Dudani A. K., Hashemi S. Characterization of plasminogen binding to human capillary and arterial endothelial cells. Biochem Cell Biol. 1991 Jul;69(7):442–448. doi: 10.1139/o91-067. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Hembrough T. A., Li L., Gonias S. L. Cell-surface cytokeratin 8 is the major plasminogen receptor on breast cancer cells and is required for the accelerated activation of cell-associated plasminogen by tissue-type plasminogen activator. J Biol Chem. 1996 Oct 11;271(41):25684–25691. doi: 10.1074/jbc.271.41.25684. [DOI] [PubMed] [Google Scholar]
- Hembrough T. A., Vasudevan J., Allietta M. M., Glass W. F., 2nd, Gonias S. L. A cytokeratin 8-like protein with plasminogen-binding activity is present on the external surfaces of hepatocytes, HepG2 cells and breast carcinoma cell lines. J Cell Sci. 1995 Mar;108(Pt 3):1071–1082. doi: 10.1242/jcs.108.3.1071. [DOI] [PubMed] [Google Scholar]
- Holst-Hansen C., Johannessen B., Høyer-Hansen G., Rømer J., Ellis V., Brünner N. Urokinase-type plasminogen activation in three human breast cancer cell lines correlates with their in vitro invasiveness. Clin Exp Metastasis. 1996 May;14(3):297–307. doi: 10.1007/BF00053903. [DOI] [PubMed] [Google Scholar]
- Jänicke F., Schmitt M., Graeff H. Clinical relevance of the urokinase-type and tissue-type plasminogen activators and of their type 1 inhibitor in breast cancer. Semin Thromb Hemost. 1991 Jul;17(3):303–312. doi: 10.1055/s-2007-1002624. [DOI] [PubMed] [Google Scholar]
- Kook Y. H., Adamski J., Zelent A., Ossowski L. The effect of antisense inhibition of urokinase receptor in human squamous cell carcinoma on malignancy. EMBO J. 1994 Sep 1;13(17):3983–3991. doi: 10.1002/j.1460-2075.1994.tb06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S., Hall R. E., Alexander I. E., Koga M., Shine J., Sutherland R. L. Inverse relationship between estrogen receptor and epidermal growth factor receptor mRNA levels in human breast cancer cell lines. Growth Factors. 1990;3(2):97–103. doi: 10.3109/08977199009108272. [DOI] [PubMed] [Google Scholar]
- Long B. J., Rose D. P. Invasive capacity and regulation of urokinase-type plasminogen activator in estrogen receptor (ER)-negative MDA-MB-231 human breast cancer cells, and a transfectant (S30) stably expressing ER. Cancer Lett. 1996 Feb 6;99(2):209–215. doi: 10.1016/0304-3835(95)04066-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Alemany R., Correc P., Camoin L., Burtin P. Purification of the plasmin receptor from human carcinoma cells and comparison to alpha-enolase. Thromb Res. 1994 Aug 15;75(4):371–381. doi: 10.1016/0049-3848(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Rifkin D. B. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993 Jan;73(1):161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- Miles L. A., Dahlberg C. M., Levin E. G., Plow E. F. Gangliosides interact directly with plasminogen and urokinase and may mediate binding of these fibrinolytic components to cells. Biochemistry. 1989 Nov 28;28(24):9337–9343. doi: 10.1021/bi00450a014. [DOI] [PubMed] [Google Scholar]
- Miles L. A., Dahlberg C. M., Plescia J., Felez J., Kato K., Plow E. F. Role of cell-surface lysines in plasminogen binding to cells: identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry. 1991 Feb 12;30(6):1682–1691. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- Milligan G., Parenti M., Magee A. I. The dynamic role of palmitoylation in signal transduction. Trends Biochem Sci. 1995 May;20(5):181–187. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- Møller L. B. Structure and function of the urokinase receptor. Blood Coagul Fibrinolysis. 1993 Apr;4(2):293–303. [PubMed] [Google Scholar]
- Nakajima K., Hamanoue M., Takemoto N., Hattori T., Kato K., Kohsaka S. Plasminogen binds specifically to alpha-enolase on rat neuronal plasma membrane. J Neurochem. 1994 Dec;63(6):2048–2057. doi: 10.1046/j.1471-4159.1994.63062048.x. [DOI] [PubMed] [Google Scholar]
- Nielsen B. S., Sehested M., Timshel S., Pyke C., Danø K. Messenger RNA for urokinase plasminogen activator is expressed in myofibroblasts adjacent to cancer cells in human breast cancer. Lab Invest. 1996 Jan;74(1):168–177. [PubMed] [Google Scholar]
- Parkkinen J., Rauvala H. Interactions of plasminogen and tissue plasminogen activator (t-PA) with amphoterin. Enhancement of t-PA-catalyzed plasminogen activation by amphoterin. J Biol Chem. 1991 Sep 5;266(25):16730–16735. [PubMed] [Google Scholar]
- Plow E. F., Miles L. A. Plasminogen receptors in the mediation of pericellular proteolysis. Cell Differ Dev. 1990 Dec 2;32(3):293–298. doi: 10.1016/0922-3371(90)90042-u. [DOI] [PubMed] [Google Scholar]
- Rana A. P., Majumder G. C. Factors influencing the yield and purity of goat sperm plasma membranes isolated by means of an aqueous two-phase polymer system. Prep Biochem. 1987;17(3):261–281. doi: 10.1080/00327488708062493. [DOI] [PubMed] [Google Scholar]
- Singleton T. P., Strickler J. G. Clinical and pathologic significance of the c-erbB-2 (HER-2/neu) oncogene. Pathol Annu. 1992;27(Pt 1):165–190. [PubMed] [Google Scholar]
- Stack M. S., Moser T. L., Pizzo S. V. Binding of human plasminogen to basement-membrane (type IV) collagen. Biochem J. 1992 May 15;284(Pt 1):103–108. doi: 10.1042/bj2840103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonelake P. S., Jones C. E., Neoptolemos J. P., Baker P. R. Proteinase inhibitors reduce basement membrane degradation by human breast cancer cell lines. Br J Cancer. 1997;75(7):951–959. doi: 10.1038/bjc.1997.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. W., Paik S., Brünner N., Sommers C. L., Zugmaier G., Clarke R., Shima T. B., Torri J., Donahue S., Lippman M. E. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992 Mar;150(3):534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]






