Abstract
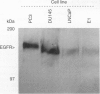
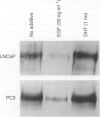
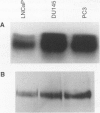
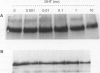
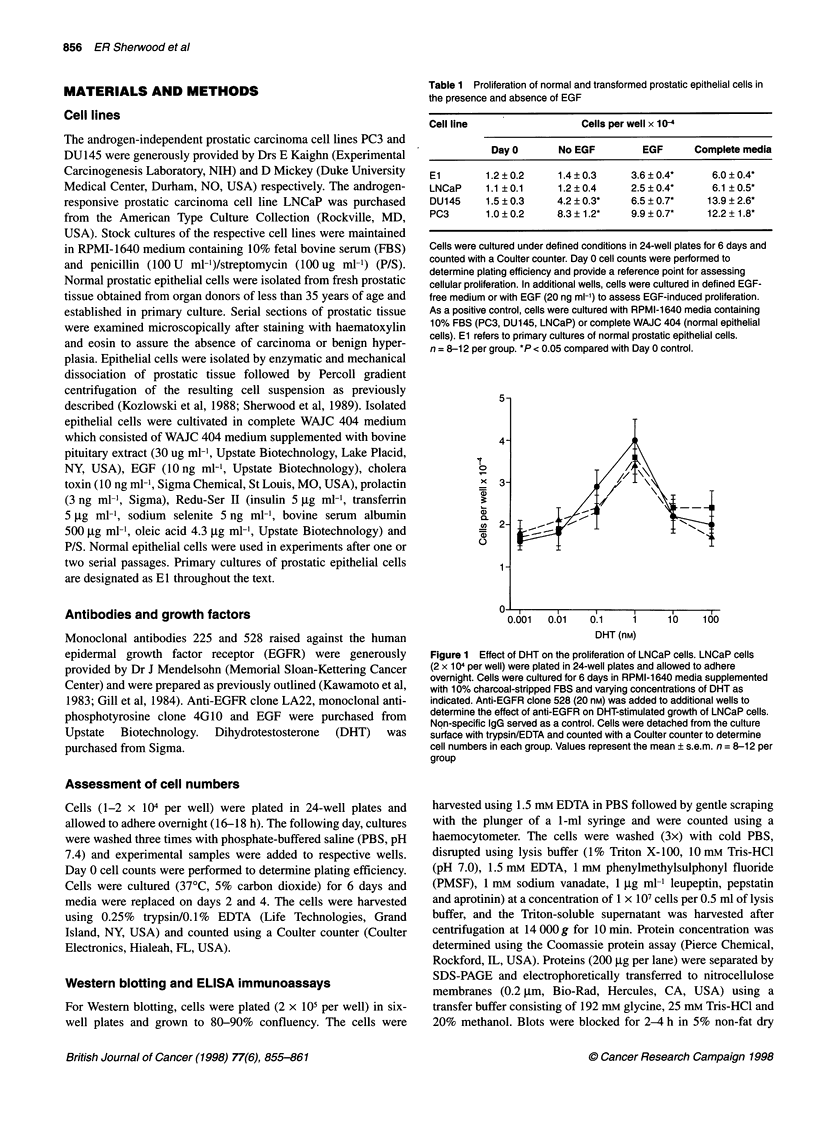
These studies were undertaken to assess the relative expression and autocrine activation of the epidermal growth factor receptor (EGFR) in normal and transformed prostatic epithelial cells and to determine whether EGFR activation plays a functional role in androgen-stimulated growth of prostate cancer cells in vitro. EGFR expression was determined by Western blot analysis and ELISA immunoassays. Immunoprecipitation of radiophosphorylated EGFR and evaluation of tyrosine phosphorylation was used to assess EGFR activation. The human androgen-independent prostate cancer cell lines PC3 and DU145 exhibited higher levels of EGFR expression and autocrine phosphorylation than normal human prostatic epithelial cells or the human androgen-responsive prostate cancer cell line LNCaP. PC3 and DU145 cells also showed higher levels of autonomous growth under serum-free defined conditions. Normal prostatic epithelial cells expressed EGFR but did not exhibit detectable levels of EGFR phosphorylation when cultured in the absence of exogenous EGF. Addition of EGF stimulated EGFR phosphorylation and induced proliferation of normal cells. LNCaP cells exhibited autocrine phosphorylation of EGFR but did not undergo significant proliferation when cultured in the absence of exogenous growth factors. A biphasic growth curve was observed when LNCaP cells were cultured with dihydrotestosterone (DHT). Maximum proliferation occurred at 1 nM DHT with regression of the growth response at DHT concentrations greater than 1 nM. However, neither EGFR expression nor phosphorylation was altered in LNCaP cells after androgen stimulation. In addition, DHT-stimulated growth of LNCaP cells was not inhibited by anti-EGFR. These studies show that autocrine activation of EGFR is a common feature of prostatic carcinoma cells in contrast to normal epithelial cells. However, EGFR activation does not appear to play a functional role in androgen-stimulated growth of LNCaP cells in vitro.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Growth factors and cancer. Science. 1991 Nov 22;254(5035):1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- BUTLER W. W., 3rd, SCHADE A. L. The effects of castration and androgen replacement on the nucleic acid composition, metabolism, and enzymatic capacities of the rat ventral prostate. Endocrinology. 1958 Sep;63(3):271–279. doi: 10.1210/endo-63-3-271. [DOI] [PubMed] [Google Scholar]
- Brass A. L., Barnard J., Patai B. L., Salvi D., Rukstalis D. B. Androgen up-regulates epidermal growth factor receptor expression and binding affinity in PC3 cell lines expressing the human androgen receptor. Cancer Res. 1995 Jul 15;55(14):3197–3203. [PubMed] [Google Scholar]
- Ching K. Z., Ramsey E., Pettigrew N., D'Cunha R., Jason M., Dodd J. G. Expression of mRNA for epidermal growth factor, transforming growth factor-alpha and their receptor in human prostate tissue and cell lines. Mol Cell Biochem. 1993 Sep 22;126(2):151–158. doi: 10.1007/BF00925693. [DOI] [PubMed] [Google Scholar]
- Connolly J. M., Rose D. P. Autocrine regulation of DU145 human prostate cancer cell growth by epidermal growth factor-related polypeptides. Prostate. 1991;19(2):173–180. doi: 10.1002/pros.2990190210. [DOI] [PubMed] [Google Scholar]
- Connolly J. M., Rose D. P. Production of epidermal growth factor and transforming growth factor-alpha by the androgen-responsive LNCaP human prostate cancer cell line. Prostate. 1990;16(3):209–218. doi: 10.1002/pros.2990160304. [DOI] [PubMed] [Google Scholar]
- Damber J. E., Bergh A., Assarsson B., Gåfvels M. Epidermal growth factor receptor content in rat prostatic adenocarcinoma: effects of endocrine treatment. Urol Res. 1995;23(2):119–125. doi: 10.1007/BF00307942. [DOI] [PubMed] [Google Scholar]
- Daneshgari F., Crawford E. D. Endocrine therapy of advanced carcinoma of the prostate. Cancer. 1993 Feb 1;71(3 Suppl):1089–1097. doi: 10.1002/1097-0142(19930201)71:3+<1089::aid-cncr2820711431>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Davies P., Eaton C. L. Binding of epidermal growth factor by human normal, hypertrophic, and carcinomatous prostate. Prostate. 1989;14(2):123–132. doi: 10.1002/pros.2990140206. [DOI] [PubMed] [Google Scholar]
- Fong C. J., Sherwood E. R., Mendelsohn J., Lee C., Kozlowski J. M. Epidermal growth factor receptor monoclonal antibody inhibits constitutive receptor phosphorylation, reduces autonomous growth, and sensitizes androgen-independent prostatic carcinoma cells to tumor necrosis factor alpha. Cancer Res. 1992 Nov 1;52(21):5887–5892. [PubMed] [Google Scholar]
- Geller J., Albert J., Vik A. Advantages of total androgen blockade in the treatment of advanced prostate cancer. Semin Oncol. 1988 Apr;15(2 Suppl 1):53–61. [PubMed] [Google Scholar]
- Gill G. N., Kawamoto T., Cochet C., Le A., Sato J. D., Masui H., McLeod C., Mendelsohn J. Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Jun 25;259(12):7755–7760. [PubMed] [Google Scholar]
- Glynne-Jones E., Goddard L., Harper M. E. Comparative analysis of mRNA and protein expression for epidermal growth factor receptor and ligands relative to the proliferative index in human prostate tissue. Hum Pathol. 1996 Jul;27(7):688–694. doi: 10.1016/s0046-8177(96)90399-8. [DOI] [PubMed] [Google Scholar]
- Ibrahim G. K., Kerns B. J., MacDonald J. A., Ibrahim S. N., Kinney R. B., Humphrey P. A., Robertson C. N. Differential immunoreactivity of epidermal growth factor receptor in benign, dysplastic and malignant prostatic tissues. J Urol. 1993 Jan;149(1):170–173. doi: 10.1016/s0022-5347(17)36032-9. [DOI] [PubMed] [Google Scholar]
- Isaacs J. T. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5(5):545–557. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- Jarrard D. F., Blitz B. F., Smith R. C., Patai B. L., Rukstalis D. B. Effect of epidermal growth factor on prostate cancer cell line PC3 growth and invasion. Prostate. 1994;24(1):46–53. doi: 10.1002/pros.2990240110. [DOI] [PubMed] [Google Scholar]
- Kaplan P. J., Leav I., Greenwood J., Kwan P. W., Ho S. M. Involvement of transforming growth factor alpha (TGFalpha) and epidermal growth factor receptor (EGFR) in sex hormone-induced prostatic dysplasia and the growth of an androgen-independent transplantable carcinoma of the prostate. Carcinogenesis. 1996 Dec;17(12):2571–2579. doi: 10.1093/carcin/17.12.2571. [DOI] [PubMed] [Google Scholar]
- Kawamoto T., Sato J. D., Le A., Polikoff J., Sato G. H., Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1337–1341. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Sensibar J. A. Proteins of the rat prostate. II. Synthesis of new proteins in the ventral lobe during castration-induced regression. J Urol. 1987 Oct;138(4):903–908. doi: 10.1016/s0022-5347(17)43413-6. [DOI] [PubMed] [Google Scholar]
- Leevers S. J., Paterson H. F., Marshall C. J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994 Jun 2;369(6479):411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- Lepor H., Ross A., Walsh P. C. The influence of hormonal therapy on survival of men with advanced prostatic cancer. J Urol. 1982 Aug;128(2):335–340. doi: 10.1016/s0022-5347(17)52915-8. [DOI] [PubMed] [Google Scholar]
- Liu X. H., Wiley H. S., Meikle A. W. Androgens regulate proliferation of human prostate cancer cells in culture by increasing transforming growth factor-alpha (TGF-alpha) and epidermal growth factor (EGF)/TGF-alpha receptor. J Clin Endocrinol Metab. 1993 Dec;77(6):1472–1478. doi: 10.1210/jcem.77.6.8263129. [DOI] [PubMed] [Google Scholar]
- MacDonald A., Habib F. K. Divergent responses to epidermal growth factor in hormone sensitive and insensitive human prostate cancer cell lines. Br J Cancer. 1992 Feb;65(2):177–182. doi: 10.1038/bjc.1992.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maygarden S. J., Strom S., Ware J. L. Localization of epidermal growth factor receptor by immunohistochemical methods in human prostatic carcinoma, prostatic intraepithelial neoplasia, and benign hyperplasia. Arch Pathol Lab Med. 1992 Mar;116(3):269–273. [PubMed] [Google Scholar]
- Mellon K., Thompson S., Charlton R. G., Marsh C., Robinson M., Lane D. P., Harris A. L., Horne C. H., Neal D. E. p53, c-erbB-2 and the epidermal growth factor receptor in the benign and malignant prostate. J Urol. 1992 Feb;147(2):496–499. doi: 10.1016/s0022-5347(17)37287-7. [DOI] [PubMed] [Google Scholar]
- Montone K. T., Tomaszewski J. E. In situ hybridization for epidermal growth factor receptor (EGFR) external domain transcripts in prostatic adenocarcinoma. J Clin Lab Anal. 1993;7(3):188–195. doi: 10.1002/jcla.1860070310. [DOI] [PubMed] [Google Scholar]
- Morris G. L., Dodd J. G. Epidermal growth factor receptor mRNA levels in human prostatic tumors and cell lines. J Urol. 1990 Jun;143(6):1272–1274. doi: 10.1016/s0022-5347(17)40253-9. [DOI] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Schuurmans A. L., Bolt J., Voorhorst M. M., Blankenstein R. A., Mulder E. Regulation of growth and epidermal growth factor receptor levels of LNCaP prostate tumor cells by different steroids. Int J Cancer. 1988 Dec 15;42(6):917–922. doi: 10.1002/ijc.2910420622. [DOI] [PubMed] [Google Scholar]
- Sherwood E. R., Berg L. A., McEwan R. N., Pasciak R. M., Kozlowski J. M., Lee C. Two-dimensional protein profiles of cultured stromal and epithelial cells from hyperplastic human prostate. J Cell Biochem. 1989 Jun;40(2):201–214. doi: 10.1002/jcb.240400209. [DOI] [PubMed] [Google Scholar]
- Turner T., Chen P., Goodly L. J., Wells A. EGF receptor signaling enhances in vivo invasiveness of DU-145 human prostate carcinoma cells. Clin Exp Metastasis. 1996 Sep;14(4):409–418. doi: 10.1007/BF00123400. [DOI] [PubMed] [Google Scholar]
- Visakorpi T., Kallioniemi O. P., Koivula T., Harvey J., Isola J. Expression of epidermal growth factor receptor and ERBB2 (HER-2/Neu) oncoprotein in prostatic carcinomas. Mod Pathol. 1992 Nov;5(6):643–648. [PubMed] [Google Scholar]
- Wilding G., Valverius E., Knabbe C., Gelmann E. P. Role of transforming growth factor-alpha in human prostate cancer cell growth. Prostate. 1989;15(1):1–12. doi: 10.1002/pros.2990150102. [DOI] [PubMed] [Google Scholar]