Abstract
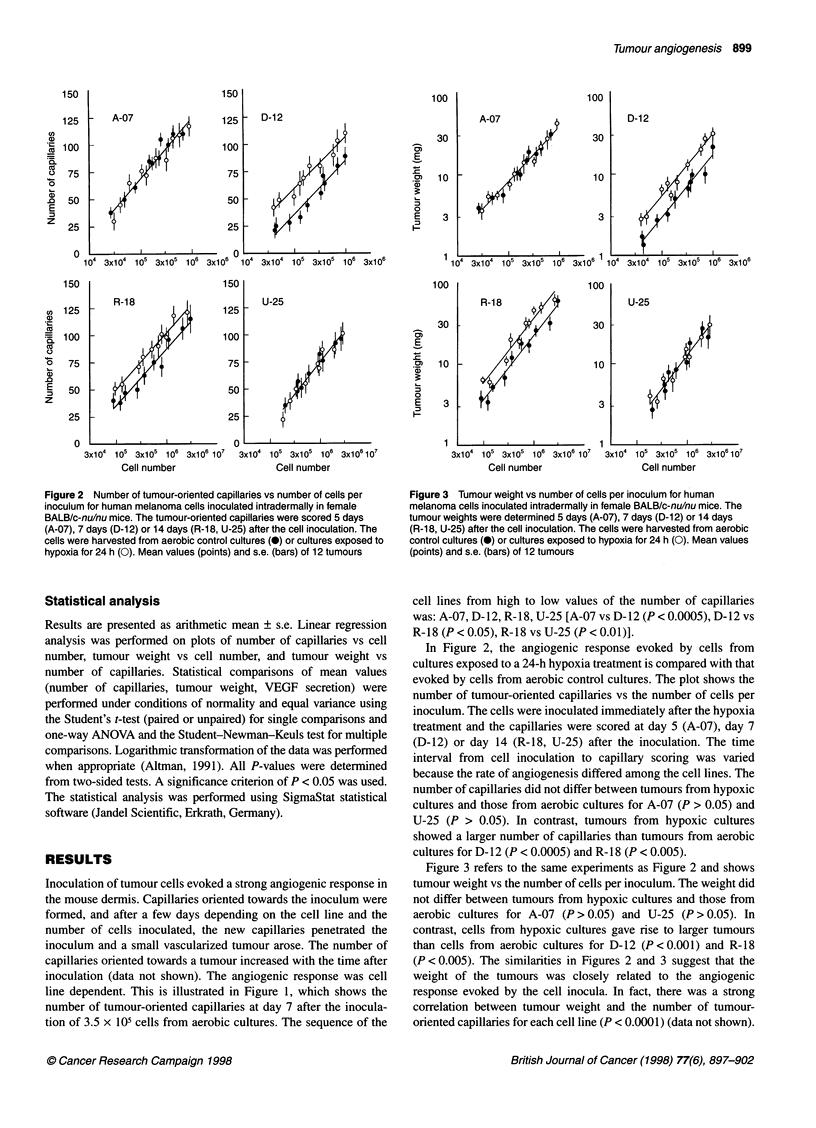
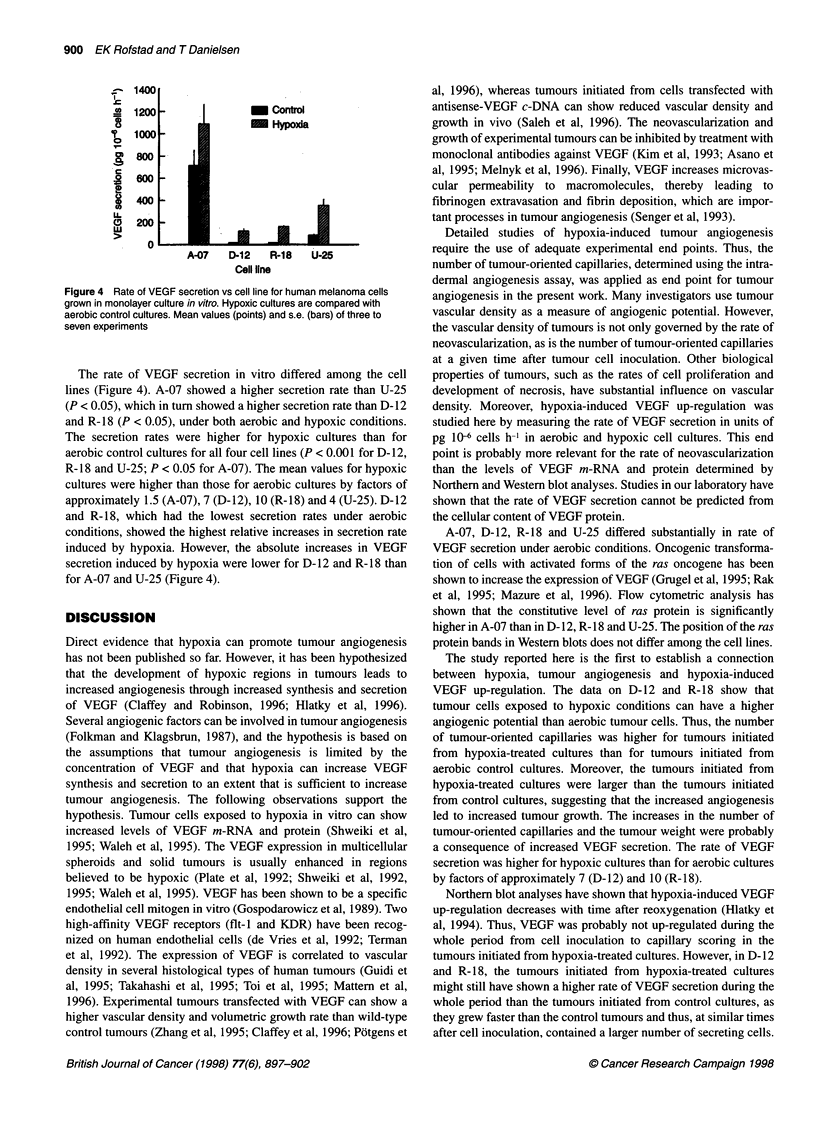
Tumour cells exposed to hypoxia in vitro can show increased expression of several selected genes, including the gene encoding the vascular endothelial growth factor (VEGF), suggesting that hypoxia followed by reoxygenation might promote the malignant progression of tumours. An in vitro/in vivo study was conducted to investigate whether hypoxia can increase the angiogenic potential of tumour cells through increased VEGF secretion. Four human melanoma cell lines (A-07, D-12, R-18, U-25) were included in the study. Cell cultures were exposed to hypoxia (oxygen concentration <10 p.p.m.) in vitro using the steel chamber method. Rate of VEGF secretion was measured in vitro in aerobic and hypoxic cell cultures by ELISA. Angiogenesis was assessed in vivo using the intradermal angiogenesis assay. Aliquots of cells harvested from aerobic cultures or cultures exposed to hypoxia for 24 h were inoculated intradermally in the flanks of adult female BALB/c-nu/nu mice. Tumours developed and angiogenesis was quantified by scoring the number of capillaries in the dermis oriented towards the tumours. The number of tumour-oriented capillaries did not differ significantly between tumours from hypoxic and aerobic cultures for A-07 and U-25, whereas tumours from hypoxic cultures showed a larger number of tumour-oriented capillaries than tumours from aerobic cultures for D-12 and R-18. The VEGF secretion under aerobic conditions and the absolute increase in VEGF secretion induced by hypoxia were lower for D-12 and R-18 than for A-07 and U-25, whereas the relative increase in VEGF secretion induced by hypoxia was higher for D-12 and R-18 than for A-07 and U-25. VEGF is not a limiting factor in the angiogenesis of some tumours under normoxic conditions. Hypoxia can increase the angiogenic potential of tumour cells by increasing the secretion of VEGF, but only of tumour cells showing low VEGF secretion under normoxia. Transient hypoxia might promote the malignant progression of tumours by temporarily increasing the angiogenic potential of tumour cells showing low VEGF expression under normoxic conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano M., Yukita A., Matsumoto T., Kondo S., Suzuki H. Inhibition of tumor growth and metastasis by an immunoneutralizing monoclonal antibody to human vascular endothelial growth factor/vascular permeability factor121. Cancer Res. 1995 Nov 15;55(22):5296–5301. [PubMed] [Google Scholar]
- Brizel D. M., Scully S. P., Harrelson J. M., Layfield L. J., Bean J. M., Prosnitz L. R., Dewhirst M. W. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996 Mar 1;56(5):941–943. [PubMed] [Google Scholar]
- Brown J. M. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979 Aug;52(620):650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Giaccia A. J. Tumour hypoxia: the picture has changed in the 1990s. Int J Radiat Biol. 1994 Jan;65(1):95–102. doi: 10.1080/09553009414550131. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Olive P. L., Durand R. E. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Res. 1987 Jan 15;47(2):597–601. [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Claffey K. P., Brown L. F., del Aguila L. F., Tognazzi K., Yeo K. T., Manseau E. J., Dvorak H. F. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996 Jan 1;56(1):172–181. [PubMed] [Google Scholar]
- Claffey K. P., Robinson G. S. Regulation of VEGF/VPF expression in tumor cells: consequences for tumor growth and metastasis. Cancer Metastasis Rev. 1996 Jun;15(2):165–176. doi: 10.1007/BF00437469. [DOI] [PubMed] [Google Scholar]
- Coleman C. N. Hypoxia in tumors: a paradigm for the approach to biochemical and physiologic heterogeneity. J Natl Cancer Inst. 1988 May 4;80(5):310–317. doi: 10.1093/jnci/80.5.310. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Ellis L. M. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994 Oct 21;79(2):185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990 Jan 3;82(1):4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Abraham J. A., Schilling J. Isolation and characterization of a vascular endothelial cell mitogen produced by pituitary-derived folliculo stellate cells. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7311–7315. doi: 10.1073/pnas.86.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grugel S., Finkenzeller G., Weindel K., Barleon B., Marmé D. Both v-Ha-Ras and v-Raf stimulate expression of the vascular endothelial growth factor in NIH 3T3 cells. J Biol Chem. 1995 Oct 27;270(43):25915–25919. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- Guidi A. J., Abu-Jawdeh G., Berse B., Jackman R. W., Tognazzi K., Dvorak H. F., Brown L. F. Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in cervical neoplasia. J Natl Cancer Inst. 1995 Aug 16;87(16):1237–1245. doi: 10.1093/jnci/87.16.1237. [DOI] [PubMed] [Google Scholar]
- Hill R. P. Tumor progression: potential role of unstable genomic changes. Cancer Metastasis Rev. 1990 Sep;9(2):137–147. doi: 10.1007/BF00046340. [DOI] [PubMed] [Google Scholar]
- Hlatky L., Tsionou C., Hahnfeldt P., Coleman C. N. Mammary fibroblasts may influence breast tumor angiogenesis via hypoxia-induced vascular endothelial growth factor up-regulation and protein expression. Cancer Res. 1994 Dec 1;54(23):6083–6086. [PubMed] [Google Scholar]
- Hockel M., Schlenger K., Aral B., Mitze M., Schaffer U., Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996 Oct 1;56(19):4509–4515. [PubMed] [Google Scholar]
- Horsman M. R. Nicotinamide and other benzamide analogs as agents for overcoming hypoxic cell radiation resistance in tumours. A review. Acta Oncol. 1995;34(5):571–587. doi: 10.3109/02841869509094031. [DOI] [PubMed] [Google Scholar]
- Kallman R. F. The phenomenon of reoxygenation and its implications for fractionated radiotherapy. Radiology. 1972 Oct;105(1):135–142. doi: 10.1148/105.1.135. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993 Apr 29;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Kreisle R. A., Ershler W. B. Investigation of tumor angiogenesis in an id mouse model: role of host-tumor interactions. J Natl Cancer Inst. 1988 Aug 3;80(11):849–854. doi: 10.1093/jnci/80.11.849. [DOI] [PubMed] [Google Scholar]
- Luk C. K., Veinot-Drebot L., Tjan E., Tannock I. F. Effect of transient hypoxia on sensitivity to doxorubicin in human and murine cell lines. J Natl Cancer Inst. 1990 Apr 18;82(8):684–692. doi: 10.1093/jnci/82.8.684. [DOI] [PubMed] [Google Scholar]
- Mattern J., Koomägi R., Volm M. Association of vascular endothelial growth factor expression with intratumoral microvessel density and tumour cell proliferation in human epidermoid lung carcinoma. Br J Cancer. 1996 Apr;73(7):931–934. doi: 10.1038/bjc.1996.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure N. M., Chen E. Y., Yeh P., Laderoute K. R., Giaccia A. J. Oncogenic transformation and hypoxia synergistically act to modulate vascular endothelial growth factor expression. Cancer Res. 1996 Aug 1;56(15):3436–3440. [PubMed] [Google Scholar]
- Melnyk O., Shuman M. A., Kim K. J. Vascular endothelial growth factor promotes tumor dissemination by a mechanism distinct from its effect on primary tumor growth. Cancer Res. 1996 Feb 15;56(4):921–924. [PubMed] [Google Scholar]
- Plate K. H., Breier G., Weich H. A., Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992 Oct 29;359(6398):845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Pötgens A. J., van Altena M. C., Lubsen N. H., Ruiter D. J., de Waal R. M. Analysis of the tumor vasculature and metastatic behavior of xenografts of human melanoma cell lines transfected with vascular permeability factor. Am J Pathol. 1996 Apr;148(4):1203–1217. [PMC free article] [PubMed] [Google Scholar]
- Rak J., Mitsuhashi Y., Bayko L., Filmus J., Shirasawa S., Sasazuki T., Kerbel R. S. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995 Oct 15;55(20):4575–4580. [PubMed] [Google Scholar]
- Rice G. C., Hoy C., Schimke R. T. Transient hypoxia enhances the frequency of dihydrofolate reductase gene amplification in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5978–5982. doi: 10.1073/pnas.83.16.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. C., Ling V., Schimke R. T. Frequencies of independent and simultaneous selection of Chinese hamster cells for methotrexate and doxorubicin (adriamycin) resistance. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9261–9264. doi: 10.1073/pnas.84.24.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofstad E. K., Johnsen N. M., Lyng H. Hypoxia-induced tetraploidisation of a diploid human melanoma cell line in vitro. Br J Cancer Suppl. 1996 Jul;27:S136–S139. [PMC free article] [PubMed] [Google Scholar]
- Rofstad E. K. Orthotopic human melanoma xenograft model systems for studies of tumour angiogenesis, pathophysiology, treatment sensitivity and metastatic pattern. Br J Cancer. 1994 Nov;70(5):804–812. doi: 10.1038/bjc.1994.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runkel S., Hunter N., Milas L. An intradermal assay for quantification and kinetics studies of tumor angiogenesis in mice. Radiat Res. 1991 May;126(2):237–243. [PubMed] [Google Scholar]
- Saleh M., Stacker S. A., Wilks A. F. Inhibition of growth of C6 glioma cells in vivo by expression of antisense vascular endothelial growth factor sequence. Cancer Res. 1996 Jan 15;56(2):393–401. [PubMed] [Google Scholar]
- Sanna K., Rofstad E. K. Hypoxia-induced resistance to doxorubicin and methotrexate in human melanoma cell lines in vitro. Int J Cancer. 1994 Jul 15;58(2):258–262. doi: 10.1002/ijc.2910580219. [DOI] [PubMed] [Google Scholar]
- Schwickert G., Walenta S., Sundfør K., Rofstad E. K., Mueller-Klieser W. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res. 1995 Nov 1;55(21):4757–4759. [PubMed] [Google Scholar]
- Senger D. R., Van de Water L., Brown L. F., Nagy J. A., Yeo K. T., Yeo T. K., Berse B., Jackman R. W., Dvorak A. M., Dvorak H. F. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993 Sep;12(3-4):303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Shweiki D., Neeman M., Itin A., Keshet E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone H. B., Brown J. M., Phillips T. L., Sutherland R. M. Oxygen in human tumors: correlations between methods of measurement and response to therapy. Summary of a workshop held November 19-20, 1992, at the National Cancer Institute, Bethesda, Maryland. Radiat Res. 1993 Dec;136(3):422–434. [PubMed] [Google Scholar]
- Takahashi Y., Kitadai Y., Bucana C. D., Cleary K. R., Ellis L. M. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995 Sep 15;55(18):3964–3968. [PubMed] [Google Scholar]
- Terman B. I., Dougher-Vermazen M., Carrion M. E., Dimitrov D., Armellino D. C., Gospodarowicz D., Böhlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- Toi M., Inada K., Hoshina S., Suzuki H., Kondo S., Tominaga T. Vascular endothelial growth factor and platelet-derived endothelial cell growth factor are frequently coexpressed in highly vascularized human breast cancer. Clin Cancer Res. 1995 Sep;1(9):961–964. [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Waleh N. S., Brody M. D., Knapp M. A., Mendonca H. L., Lord E. M., Koch C. J., Laderoute K. R., Sutherland R. M. Mapping of the vascular endothelial growth factor-producing hypoxic cells in multicellular tumor spheroids using a hypoxia-specific marker. Cancer Res. 1995 Dec 15;55(24):6222–6226. [PubMed] [Google Scholar]
- Walenta S., Salameh A., Lyng H., Evensen J. F., Mitze M., Rofstad E. K., Mueller-Klieser W. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol. 1997 Feb;150(2):409–415. [PMC free article] [PubMed] [Google Scholar]
- Wilson R. E., Keng P. C., Sutherland R. M. Changes in growth characteristics and macromolecular synthesis on recovery from severe hypoxia. Br J Cancer. 1990 Jan;61(1):14–21. doi: 10.1038/bjc.1990.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. D., Marshall R. S., Hill R. P. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9533–9537. doi: 10.1073/pnas.85.24.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagzag D. Angiogenic growth factors in neural embryogenesis and neoplasia. Am J Pathol. 1995 Feb;146(2):293–309. [PMC free article] [PubMed] [Google Scholar]
- Zhang H. T., Craft P., Scott P. A., Ziche M., Weich H. A., Harris A. L., Bicknell R. Enhancement of tumor growth and vascular density by transfection of vascular endothelial cell growth factor into MCF-7 human breast carcinoma cells. J Natl Cancer Inst. 1995 Feb 1;87(3):213–219. doi: 10.1093/jnci/87.3.213. [DOI] [PubMed] [Google Scholar]
- de Vries C., Escobedo J. A., Ueno H., Houck K., Ferrara N., Williams L. T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992 Feb 21;255(5047):989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]


