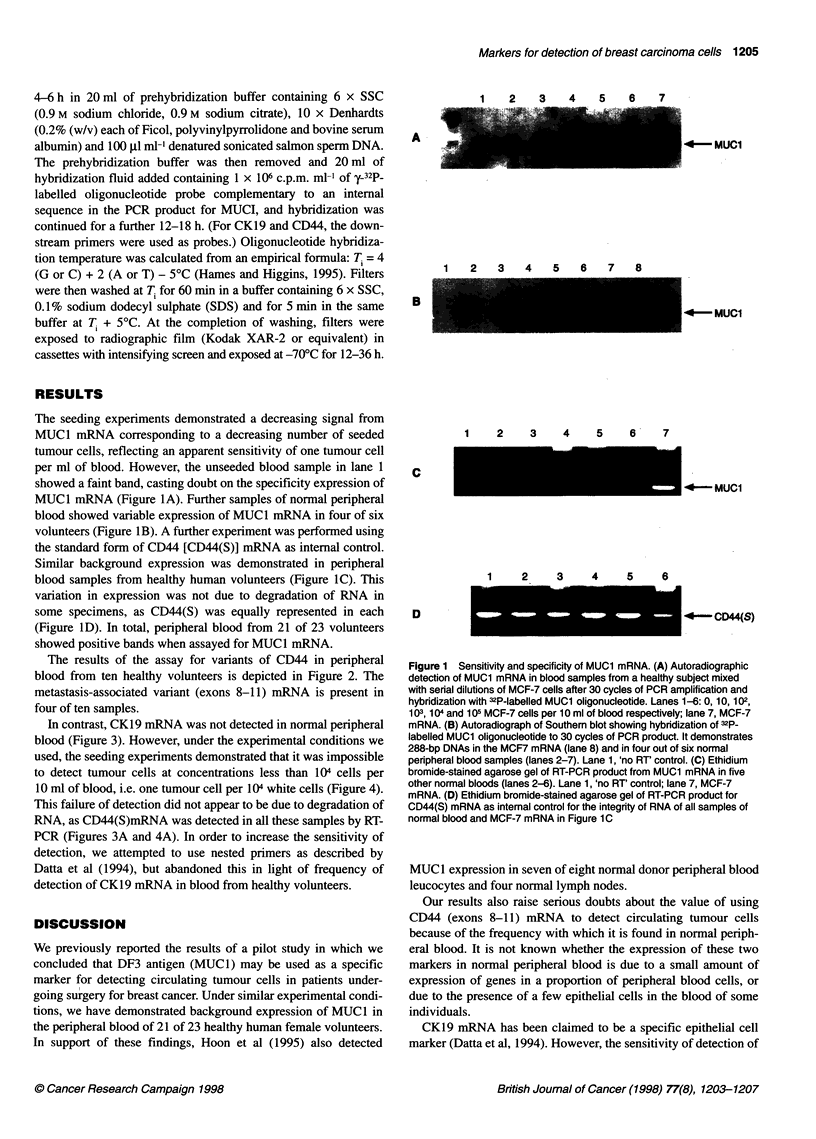
Abstract
The aim of this study was to investigate certain genes for their suitability as molecular markers for detection of breast carcinoma cells using the reverse transcriptase-polymerase chain reaction (RT-PCR). RNA was prepared from MCF-7 breast carcinoma cells and peripheral blood leucocytes of healthy female volunteers. This RNA was screened for mRNA of MUC1, cytokeratin 19 (CK19) and CD44 (exons 8-11) by RT-PCR and the results validated by Southern blots. Variable degrees of expression of MUC1 and CD44 (exons 8-11) were detected in normal peripheral blood, rendering these genes non-specific for epithelial cells and therefore unsuitable for use as markers to detect breast carcinoma cells. Although CK19 mRNA was apparently specific, it was deemed unsuitable for use as a marker of breast cancer cells in light of its limited sensitivity. Furthermore, an attempt at using nested primers to increase sensitivity resulted in CK19 mRNA being detected after two amplification rounds in blood from healthy volunteers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Kufe D. Characterization of cis-acting elements regulating transcription of the human DF3 breast carcinoma-associated antigen (MUC1) gene. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):282–286. doi: 10.1073/pnas.90.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. C., Purushotham A. D., Birnie G. D., George W. D. Detection of intraoperative tumor cell dissemination in patients with breast cancer by use of reverse transcription and polymerase chain reaction. Surgery. 1995 Jan;117(1):95–101. doi: 10.1016/s0039-6060(05)80235-1. [DOI] [PubMed] [Google Scholar]
- Burchill S. A., Bradbury F. M., Smith B., Lewis I. J., Selby P. Neuroblastoma cell detection by reverse transcriptase-polymerase chain reaction (RT-PCR) for tyrosine hydroxylase mRNA. Int J Cancer. 1994 Jun 1;57(5):671–675. doi: 10.1002/ijc.2910570510. [DOI] [PubMed] [Google Scholar]
- Chelly J., Concordet J. P., Kaplan J. C., Kahn A. Illegitimate transcription: transcription of any gene in any cell type. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2617–2621. doi: 10.1073/pnas.86.8.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy A., McCulloch P. Induction of tumour cell shedding into effluent venous blood breast cancer surgery. Br J Cancer. 1996 Jan;73(1):79–82. doi: 10.1038/bjc.1996.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta Y. H., Adams P. T., Drobyski W. R., Ethier S. P., Terry V. H., Roth M. S. Sensitive detection of occult breast cancer by the reverse-transcriptase polymerase chain reaction. J Clin Oncol. 1994 Mar;12(3):475–482. doi: 10.1200/JCO.1994.12.3.475. [DOI] [PubMed] [Google Scholar]
- Diel I. J., Kaufmann M., Goerner R., Costa S. D., Kaul S., Bastert G. Detection of tumor cells in bone marrow of patients with primary breast cancer: a prognostic factor for distant metastasis. J Clin Oncol. 1992 Oct;10(10):1534–1539. doi: 10.1200/JCO.1992.10.10.1534. [DOI] [PubMed] [Google Scholar]
- Dilworth D. D., McCarrey J. R. Single-step elimination of contaminating DNA prior to reverse transcriptase PCR. PCR Methods Appl. 1992 May;1(4):279–282. doi: 10.1101/gr.1.4.279. [DOI] [PubMed] [Google Scholar]
- Eschwège P., Dumas F., Blanchet P., Le Maire V., Benoit G., Jardin A., Lacour B., Loric S. Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet. 1995 Dec 9;346(8989):1528–1530. doi: 10.1016/s0140-6736(95)92054-4. [DOI] [PubMed] [Google Scholar]
- Feuer E. J., Wun L. M., Boring C. C., Flanders W. D., Timmel M. J., Tong T. The lifetime risk of developing breast cancer. J Natl Cancer Inst. 1993 Jun 2;85(11):892–897. doi: 10.1093/jnci/85.11.892. [DOI] [PubMed] [Google Scholar]
- Fox S. B., Fawcett J., Jackson D. G., Collins I., Gatter K. C., Harris A. L., Gearing A., Simmons D. L. Normal human tissues, in addition to some tumors, express multiple different CD44 isoforms. Cancer Res. 1994 Aug 15;54(16):4539–4546. [PubMed] [Google Scholar]
- Frank J. A., Ling A., Patronas N. J., Carrasquillo J. A., Horvath K., Hickey A. M., Dwyer A. J. Detection of malignant bone tumors: MR imaging vs scintigraphy. AJR Am J Roentgenol. 1990 Nov;155(5):1043–1048. doi: 10.2214/ajr.155.5.2120933. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Verwer B., Houck D., Hoffman R. A., Recktenwald D. Model study detecting breast cancer cells in peripheral blood mononuclear cells at frequencies as low as 10(-7). Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):537–541. doi: 10.1073/pnas.92.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Hardingham J. E., Kotasek D., Farmer B., Butler R. N., Mi J. X., Sage R. E., Dobrovic A. Immunobead-PCR: a technique for the detection of circulating tumor cells using immunomagnetic beads and the polymerase chain reaction. Cancer Res. 1993 Aug 1;53(15):3455–3458. [PubMed] [Google Scholar]
- Hardingham J. E., Kotasek D., Sage R. E., Eaton M. C., Pascoe V. H., Dobrovic A. Detection of circulating tumor cells in colorectal cancer by immunobead-PCR is a sensitive prognostic marker for relapse of disease. Mol Med. 1995 Nov;1(7):789–794. [PMC free article] [PubMed] [Google Scholar]
- Ho S. B., Niehans G. A., Lyftogt C., Yan P. S., Cherwitz D. L., Gum E. T., Dahiya R., Kim Y. S. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993 Feb 1;53(3):641–651. [PubMed] [Google Scholar]
- Hoon D. S., Doi F., Giuliano A. E., Schmid P., Conrad A. J. The detection of breast carcinoma micrometastases in axillary lymph nodes by means of reverse transcriptase-polymerase chain reaction. Cancer. 1995 Aug 1;76(3):533–535. doi: 10.1002/1097-0142(19950801)76:3<533::aid-cncr2820760330>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Johnson P. W., Burchill S. A., Selby P. J. The molecular detection of circulating tumour cells. Br J Cancer. 1995 Aug;72(2):268–276. doi: 10.1038/bjc.1995.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y., Tarin D. Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet. 1992 Oct 31;340(8827):1053–1058. doi: 10.1016/0140-6736(92)93077-z. [DOI] [PubMed] [Google Scholar]
- McCulloch P., Choy A., Martin L. Association between tumour angiogenesis and tumour cell shedding into effluent venous blood during breast cancer surgery. Lancet. 1995 Nov 18;346(8986):1334–1335. doi: 10.1016/s0140-6736(95)92345-4. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Screaton G. R., Bell M. V., Jackson D. G., Cornelis F. B., Gerth U., Bell J. I. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B., Selby P., Southgate J., Pittman K., Bradley C., Blair G. E. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet. 1991 Nov 16;338(8777):1227–1229. doi: 10.1016/0140-6736(91)92100-g. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Steward C. G., Goulden N. J., Katz F., Baines D., Martin P. G., Langlands K., Potter M. N., Chessells J. M., Oakhill A. A polymerase chain reaction study of the stability of Ig heavy-chain and T-cell receptor delta gene rearrangements between presentation and relapse of childhood B-lineage acute lymphoblastic leukemia. Blood. 1994 Mar 1;83(5):1355–1362. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Berdichevsky F., D'Souza B., Burchell J. Human models of breast cancer. Cancer Surv. 1993;16:59–78. [PubMed] [Google Scholar]
- Yamada M., Wasserman R., Lange B., Reichard B. A., Womer R. B., Rovera G. Minimal residual disease in childhood B-lineage lymphoblastic leukemia. Persistence of leukemic cells during the first 18 months of treatment. N Engl J Med. 1990 Aug 16;323(7):448–455. doi: 10.1056/NEJM199008163230705. [DOI] [PubMed] [Google Scholar]
- de Graaf H., Maelandsmo G. M., Ruud P., Forus A., Oyjord T., Fodstad O., Hovig E. Ectopic expression of target genes may represent an inherent limitation of RT-PCR assays used for micrometastasis detection: studies on the epithelial glycoprotein gene EGP-2. Int J Cancer. 1997 Jul 3;72(1):191–196. doi: 10.1002/(sici)1097-0215(19970703)72:1<191::aid-ijc27>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- de la Torre M., Heldin P., Bergh J. Expression of the CD44 glycoprotein (lymphocyte-homing receptor) in untreated human breast cancer and its relationship to prognostic markers. Anticancer Res. 1995 Nov-Dec;15(6B):2791–2795. [PubMed] [Google Scholar]