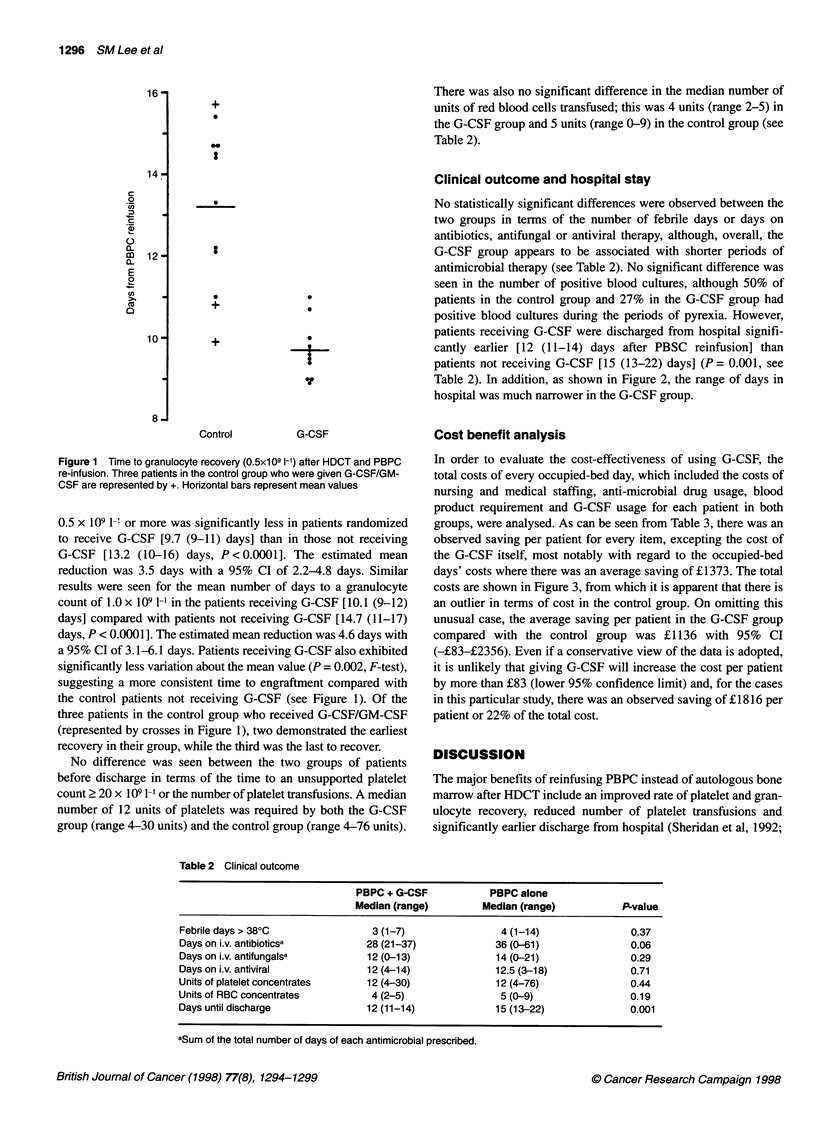
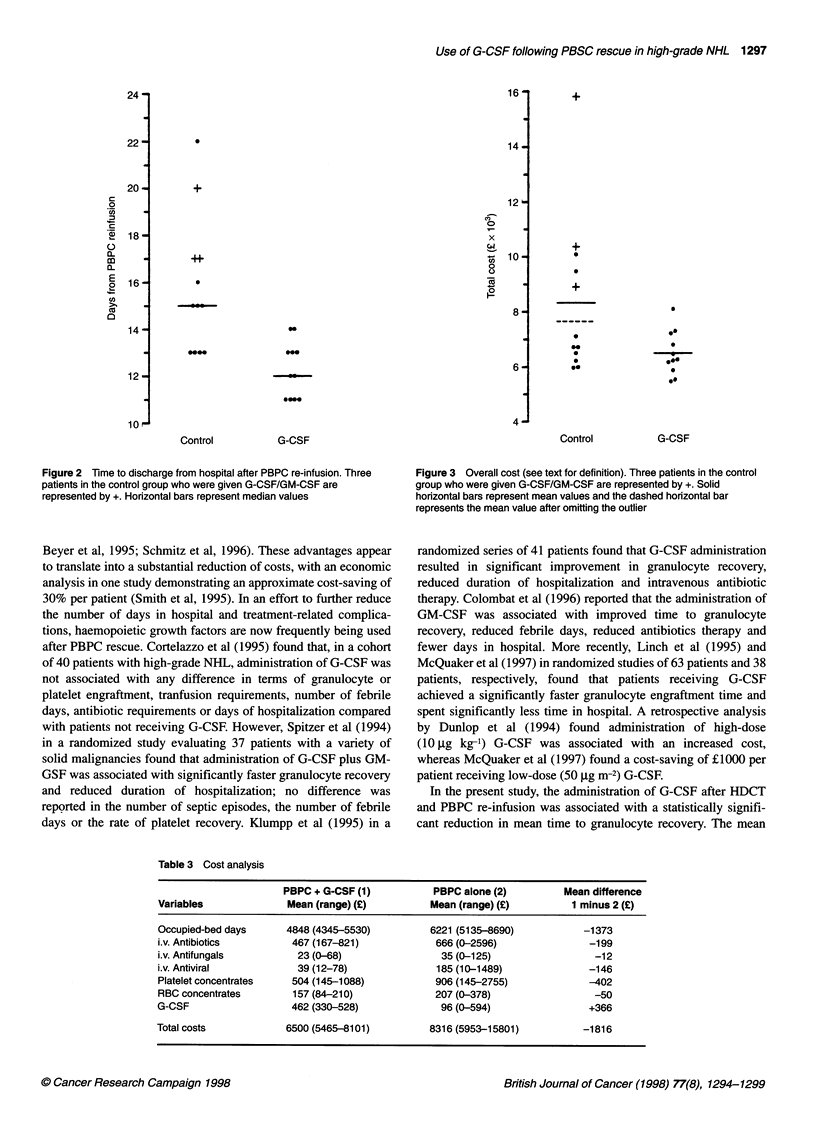
Abstract
In order to evaluate the potential clinical and economic benefits of granulocyte colony-stimulating factor (G-CSF, filgrastim) following peripheral blood progenitor cells (PBPC) rescue after high-dose chemotherapy (HDCT), 23 consecutive patients aged less than 60 years with poor-prognosis, high-grade non-Hodgkin's lymphoma (NHL) were entered into a prospective randomized trial between May 1993 and September 1995. Patients were randomized to receive either PBPC alone (n = 12) or PBPC+G-CSF (n = 11) after HDCT with busulphan and cyclophosphamide. G-CSF (300 microg day[-1]) was given from day +5 until recovery of granulocyte count to greater than 1.0 x 10(9) l(-1) for 2 consecutive days. The mean time to achieve a granulocyte count > 0.5 x 10(9) l(-1) was significantly shorter in the G-CSF arm (9.7 vs 13.2 days; P<0.0001) as was the median duration of hospital stay (12 vs 15 days; P = 0.001). In addition the recovery periods (range 9-12 vs 11-17 days to achieve a count of 1.0 x 10(9) l[-1]) and hospital stays (range 11-14 vs 13-22 days) were significantly less variable in patients receiving G-CSF in whom the values clustered around the median. There were no statistically significant differences between the study arms in terms of days of fever, documented episodes of bacteraemia, antimicrobial drug usage and platelet/red cell transfusion requirements. Taking into account the costs of total occupied-bed days, drugs, growth factor usage and haematological support, the mean expenditure per inpatient stay was pound sterling 6500 (range pound sterling 5465-pound sterling 8101) in the G-CSF group compared with pound sterling 8316 (range pound sterling 5953-pound sterling 15,801) in the group not receiving G-CSF, with an observed mean saving of 1816 per patient (or 22% of the total cost) in the G-CSF group. This study suggests that after HDCT and PBPC rescue, the use of G-CSF leads to more rapid haematological recovery periods and is associated with a more predictable and shorter hospital stay. Furthermore, and despite the additional costs for G-CSF, these clinical benefits are not translated into increased health care expenditure.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer J., Schwella N., Zingsem J., Strohscheer I., Schwaner I., Oettle H., Serke S., Huhn D., Stieger W. Hematopoietic rescue after high-dose chemotherapy using autologous peripheral-blood progenitor cells or bone marrow: a randomized comparison. J Clin Oncol. 1995 Jun;13(6):1328–1335. doi: 10.1200/JCO.1995.13.6.1328. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Buckley M., Sathe Y. S., Freireich E. J. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966 Feb;64(2):328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- Colombat P., Delain M., Desbois I., Domenech J., Binet C., Tabah I., Lamagnere J. P., Linassier C. Granulocyte-macrophage colony-stimulating factor accelerates hematopoietic recovery after autologous bone marrow or peripheral blood progenitor cell transplantation and high-dose chemotherapy for lymphoma. Bone Marrow Transplant. 1996 Aug;18(2):293–299. [PubMed] [Google Scholar]
- Cortelazzo S., Viero P., Bellavita P., Rossi A., Buelli M., Borleri G. M., Marziali S., Bassan R., Comotti B., Rambaldi A. Granulocyte colony-stimulating factor following peripheral-blood progenitor-cell transplant in non-Hodgkin's lymphoma. J Clin Oncol. 1995 Apr;13(4):935–941. doi: 10.1200/JCO.1995.13.4.935. [DOI] [PubMed] [Google Scholar]
- Dunlop D. J., Fitzsimons E. J., McMurray A., Morrison M., Kyle E., Alcorn M. J., Steward W. P. Filgrastim fails to improve haemopoietic reconstitution following myeloablative chemotherapy and peripheral blood stem cell rescue. Br J Cancer. 1994 Nov;70(5):943–945. doi: 10.1038/bjc.1994.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselbrecht C., Prentice H. G., Bacigalupo A., Biron P., Milpied N., Rubie H., Cunningham D., Legros M., Pico J. L., Linch D. C. Placebo-controlled phase III trial of lenograstim in bone-marrow transplantation. Lancet. 1994 Mar 19;343(8899):696–700. doi: 10.1016/s0140-6736(94)91579-2. [DOI] [PubMed] [Google Scholar]
- Klumpp T. R., Mangan K. F., Goldberg S. L., Pearlman E. S., Macdonald J. S. Granulocyte colony-stimulating factor accelerates neutrophil engraftment following peripheral-blood stem-cell transplantation: a prospective, randomized trial. J Clin Oncol. 1995 Jun;13(6):1323–1327. doi: 10.1200/JCO.1995.13.6.1323. [DOI] [PubMed] [Google Scholar]
- Linch D. C., Scarffe H., Proctor S., Chopra R., Taylor P. R., Morgenstern G., Cunningham D., Burnett A. K., Cawley J. C., Franklin I. M. Randomised vehicle-controlled dose-finding study of glycosylated recombinant human granulocyte colony-stimulating factor after bone marrow transplantation. Bone Marrow Transplant. 1993 Apr;11(4):307–311. [PubMed] [Google Scholar]
- Link H., Boogaerts M. A., Carella A. M., Ferrant A., Gadner H., Gorin N. C., Harabacz I., Harousseau J. L., Hervé P., Holldack J. A controlled trial of recombinant human granulocyte-macrophage colony-stimulating factor after total body irradiation, high-dose chemotherapy, and autologous bone marrow transplantation for acute lymphoblastic leukemia or malignant lymphoma. Blood. 1992 Nov 1;80(9):2188–2195. [PubMed] [Google Scholar]
- McQuaker I. G., Hunter A. E., Pacey S., Haynes A. P., Iqbal A., Russell N. H. Low-dose filgrastim significantly enhances neutrophil recovery following autologous peripheral-blood stem-cell transplantation in patients with lymphoproliferative disorders: evidence for clinical and economic benefit. J Clin Oncol. 1997 Feb;15(2):451–457. doi: 10.1200/JCO.1997.15.2.451. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Proliferative effects of purified granulocyte colony-stimulating factor (G-CSF) on normal mouse hemopoietic cells. J Cell Physiol. 1983 Aug;116(2):198–206. doi: 10.1002/jcp.1041160211. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J., Rabinowe S. N., Singer J. W., Bierman P. J., Vose J. M., Freedman A. S., Onetto N., Gillis S., Oette D., Gold M. Recombinant granulocyte-macrophage colony-stimulating factor after autologous bone marrow transplantation for lymphoid cancer. N Engl J Med. 1991 Jun 20;324(25):1773–1778. doi: 10.1056/NEJM199106203242504. [DOI] [PubMed] [Google Scholar]
- Peters W. P., Rosner G., Ross M., Vredenburgh J., Meisenberg B., Gilbert C., Kurtzberg J. Comparative effects of granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) on priming peripheral blood progenitor cells for use with autologous bone marrow after high-dose chemotherapy. Blood. 1993 Apr 1;81(7):1709–1719. [PubMed] [Google Scholar]
- Pettengell R., Radford J. A., Morgenstern G. R., Scarffe J. H., Harris M., Woll P. J., Deakin D. P., Ryder D., Wilkinson P. M., Crowther D. Survival benefit from high-dose therapy with autologous blood progenitor-cell transplantation in poor-prognosis non-Hodgkin's lymphoma. J Clin Oncol. 1996 Feb;14(2):586–592. doi: 10.1200/JCO.1996.14.2.586. [DOI] [PubMed] [Google Scholar]
- Schmitz N., Linch D. C., Dreger P., Goldstone A. H., Boogaerts M. A., Ferrant A., Demuynck H. M., Link H., Zander A., Barge A. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996 Feb 10;347(8998):353–357. doi: 10.1016/s0140-6736(96)90536-x. [DOI] [PubMed] [Google Scholar]
- Sheridan W. P., Morstyn G., Wolf M., Dodds A., Lusk J., Maher D., Layton J. E., Green M. D., Souza L., Fox R. M. Granulocyte colony-stimulating factor and neutrophil recovery after high-dose chemotherapy and autologous bone marrow transplantation. Lancet. 1989 Oct 14;2(8668):891–895. doi: 10.1016/s0140-6736(89)91552-3. [DOI] [PubMed] [Google Scholar]
- Shimazaki C., Oku N., Uchiyama H., Yamagata N., Tatsumi T., Hirata T., Ashihara E., Okawa K., Goto H., Inaba T. Effect of granulocyte colony-stimulating factor on hematopoietic recovery after peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 1994 Mar;13(3):271–275. [PubMed] [Google Scholar]
- Spitzer G., Adkins D. R., Spencer V., Dunphy F. R., Petruska P. J., Velasquez W. S., Bowers C. E., Kronmueller N., Niemeyer R., McIntyre W. Randomized study of growth factors post-peripheral-blood stem-cell transplant: neutrophil recovery is improved with modest clinical benefit. J Clin Oncol. 1994 Apr;12(4):661–670. doi: 10.1200/JCO.1994.12.4.661. [DOI] [PubMed] [Google Scholar]
- Stahel R. A., Jost L. M., Cerny T., Pichert G., Honegger H., Tobler A., Jacky E., Fey M., Platzer E. Randomized study of recombinant human granulocyte colony-stimulating factor after high-dose chemotherapy and autologous bone marrow transplantation for high-risk lymphoid malignancies. J Clin Oncol. 1994 Sep;12(9):1931–1938. doi: 10.1200/JCO.1994.12.9.1931. [DOI] [PubMed] [Google Scholar]
- Torres Gómez A., Jimenez M. A., Alvarez M. A., Rodriguez A., Martin C., Garcia M. J., Flores R., Sanchez J., de la Torre M. J., Herrera C. Optimal timing of granulocyte colony-stimulating factor (G-CSF) administration after bone marrow transplantation. A prospective randomized study. Ann Hematol. 1995 Aug;71(2):65–70. doi: 10.1007/BF01699248. [DOI] [PubMed] [Google Scholar]


