Abstract
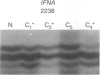
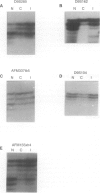
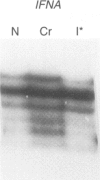
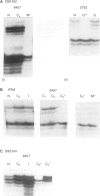
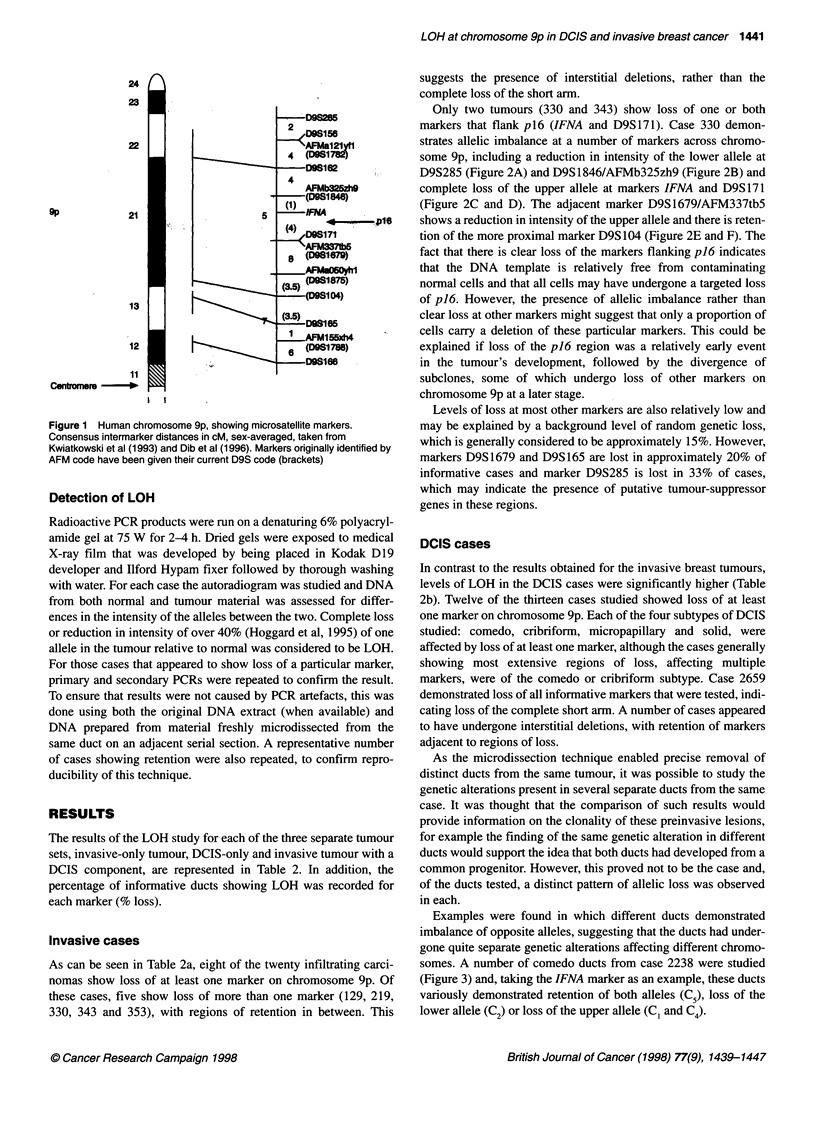
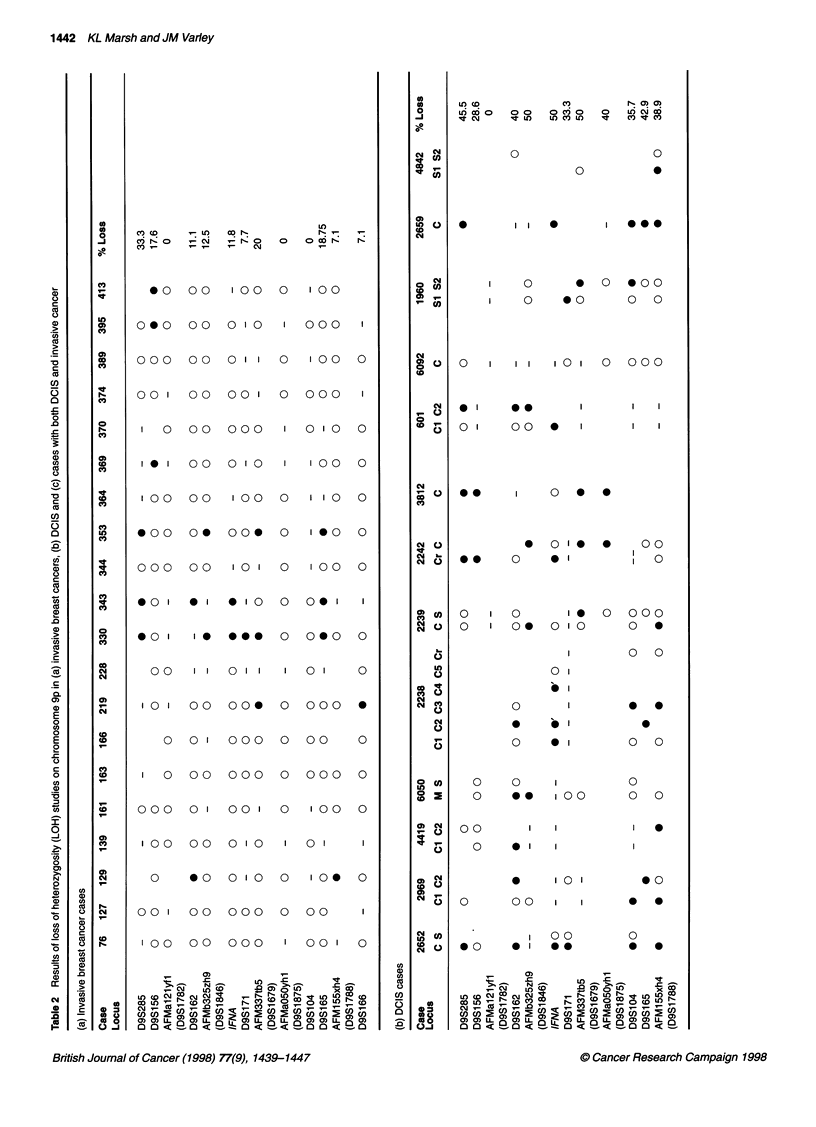
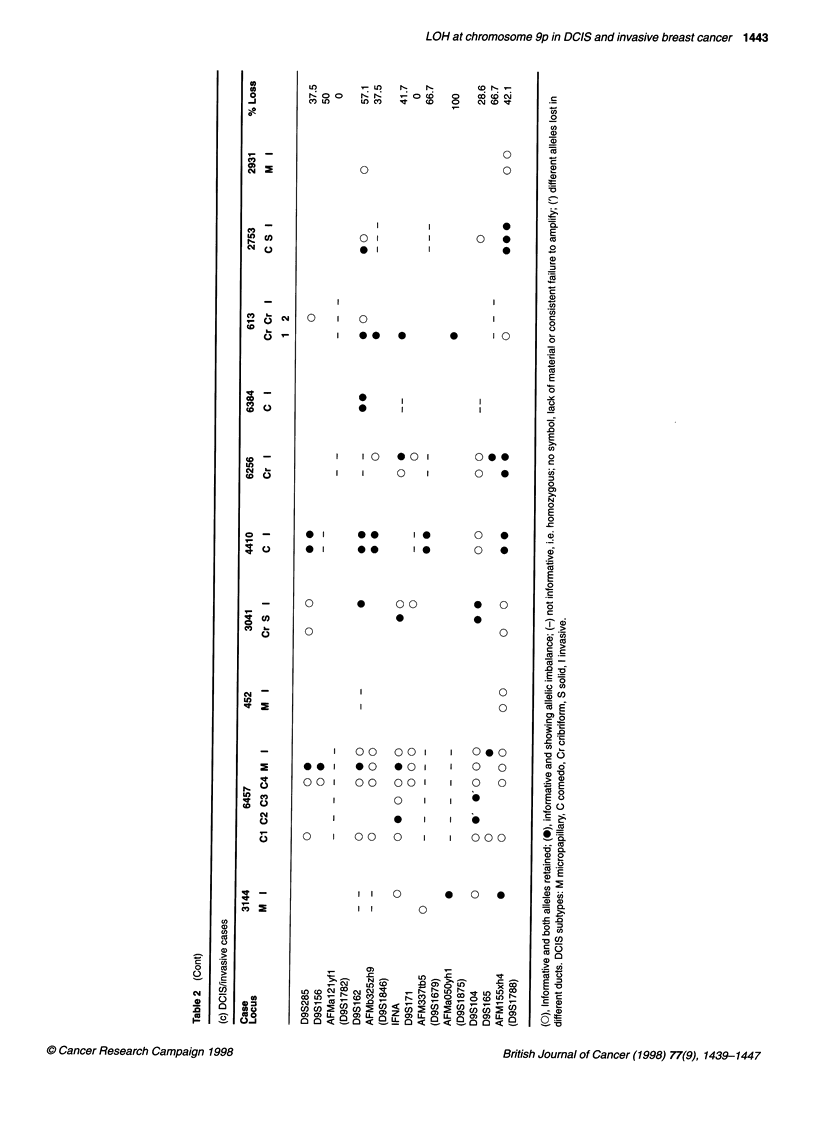
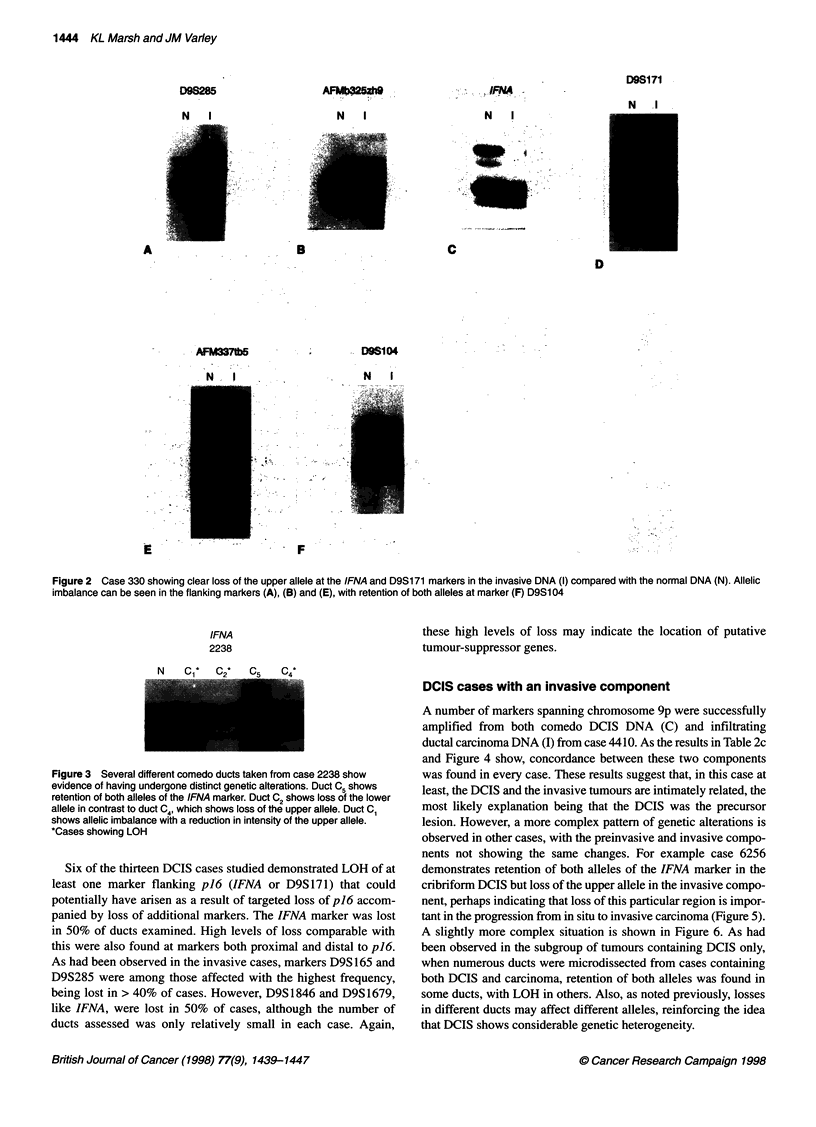
Twenty-three cases of ductal carcinoma in situ (DCIS), ten of which had an associated invasive component, were studied for loss of heterozygosity (LOH) of microsatellite markers on chromosome 9p and the results compared with a panel of 20 invasive breast carcinomas. In addition to the gene encoding p16, chromosome 9p is also thought to contain other putative tumour-suppressor genes. If the three panels of breast tumours showed LOH of markers in this region this would suggest that such putative genes were important in breast carcinogenesis. By studying both preinvasive and invasive breast tumours, it should also be possible to gain further information about the relationship between lesions of a different stage and to determine whether DCIS is indeed a precursor of invasive ductal carcinoma. Levels of LOH were low in the invasive-only set of tumours. Surprisingly, considerably higher levels of loss were observed in the tumours with an in situ component. Also, much heterogeneity was observed between different DCIS ducts or invasive tumour and DCIS from the same case.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldaz C. M., Chen T., Sahin A., Cunningham J., Bondy M. Comparative allelotype of in situ and invasive human breast cancer: high frequency of microsatellite instability in lobular breast carcinomas. Cancer Res. 1995 Sep 15;55(18):3976–3981. [PubMed] [Google Scholar]
- Barnes D. M., Bartkova J., Camplejohn R. S., Gullick W. J., Smith P. J., Millis R. R. Overexpression of the c-erbB-2 oncoprotein: why does this occur more frequently in ductal carcinoma in situ than in invasive mammary carcinoma and is this of prognostic significance? Eur J Cancer. 1992;28(2-3):644–648. doi: 10.1016/s0959-8049(05)80117-0. [DOI] [PubMed] [Google Scholar]
- Bonsing B. A., Devilee P., Cleton-Jansen A. M., Kuipers-Dijkshoorn N., Fleuren G. J., Cornelisse C. J. Evidence for limited molecular genetic heterogeneity as defined by allelotyping and clonal analysis in nine metastatic breast carcinomas. Cancer Res. 1993 Aug 15;53(16):3804–3811. [PubMed] [Google Scholar]
- Cairns P., Tokino K., Eby Y., Sidransky D. Localization of tumor suppressor loci on chromosome 9 in primary human renal cell carcinomas. Cancer Res. 1995 Jan 15;55(2):224–227. [PubMed] [Google Scholar]
- Callahan R., Cropp C., Sheng Z. M., Merlo G., Steeg P., Liscia D., Lidereau R. Definition of regions of the human genome affected by loss of heterozygosity in primary human breast tumors. J Cell Biochem Suppl. 1993;17G:167–172. doi: 10.1002/jcb.240531131. [DOI] [PubMed] [Google Scholar]
- Chen L. C., Kurisu W., Ljung B. M., Goldman E. S., Moore D., 2nd, Smith H. S. Heterogeneity for allelic loss in human breast cancer. J Natl Cancer Inst. 1992 Apr 1;84(7):506–510. doi: 10.1093/jnci/84.7.506. [DOI] [PubMed] [Google Scholar]
- Coleman A., Fountain J. W., Nobori T., Olopade O. I., Robertson G., Housman D. E., Lugo T. G. Distinct deletions of chromosome 9p associated with melanoma versus glioma, lung cancer, and leukemia. Cancer Res. 1994 Jan 15;54(2):344–348. [PubMed] [Google Scholar]
- Deng G., Lu Y., Zlotnikov G., Thor A. D., Smith H. S. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996 Dec 20;274(5295):2057–2059. doi: 10.1126/science.274.5295.2057. [DOI] [PubMed] [Google Scholar]
- Devlin J., Keen A. J., Knowles M. A. Homozygous deletion mapping at 9p21 in bladder carcinoma defines a critical region within 2cM of IFNA. Oncogene. 1994 Sep;9(9):2757–2760. [PubMed] [Google Scholar]
- Dib C., Fauré S., Fizames C., Samson D., Drouot N., Vignal A., Millasseau P., Marc S., Hazan J., Seboun E. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature. 1996 Mar 14;380(6570):152–154. doi: 10.1038/380152a0. [DOI] [PubMed] [Google Scholar]
- Fujii H., Marsh C., Cairns P., Sidransky D., Gabrielson E. Genetic divergence in the clonal evolution of breast cancer. Cancer Res. 1996 Apr 1;56(7):1493–1497. [PubMed] [Google Scholar]
- Gruis N. A., van der Velden P. A., Sandkuijl L. A., Prins D. E., Weaver-Feldhaus J., Kamb A., Bergman W., Frants R. R. Homozygotes for CDKN2 (p16) germline mutation in Dutch familial melanoma kindreds. Nat Genet. 1995 Jul;10(3):351–353. doi: 10.1038/ng0795-351. [DOI] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Hoggard N., Brintnell B., Howell A., Weissenbach J., Varley J. Allelic imbalance on chromosome 1 in human breast cancer. II. Microsatellite repeat analysis. Genes Chromosomes Cancer. 1995 Jan;12(1):24–31. doi: 10.1002/gcc.2870120105. [DOI] [PubMed] [Google Scholar]
- Hussussian C. J., Struewing J. P., Goldstein A. M., Higgins P. A., Ally D. S., Sheahan M. D., Clark W. H., Jr, Tucker M. A., Dracopoli N. C. Germline p16 mutations in familial melanoma. Nat Genet. 1994 Sep;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- Ichimura K., Schmidt E. E., Yamaguchi N., James C. D., Collins V. P. A common region of homozygous deletion in malignant human gliomas lies between the IFN alpha/omega gene cluster and the D9S171 locus. Cancer Res. 1994 Jun 15;54(12):3127–3130. [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Diaz M. O. Dinucleotide repeat polymorphism at the IFNA locus (9p22). Hum Mol Genet. 1992 Nov;1(8):658–658. doi: 10.1093/hmg/1.8.658-a. [DOI] [PubMed] [Google Scholar]
- Lagios M. D. Heterogeneity of ductal carcinoma in situ of the breast. J Cell Biochem Suppl. 1993;17G:49–52. [PubMed] [Google Scholar]
- Mead L. J., Gillespie M. T., Irving L. B., Campbell L. J. Homozygous and hemizygous deletions of 9p centromeric to the interferon genes in lung cancer. Cancer Res. 1994 May 1;54(9):2307–2309. [PubMed] [Google Scholar]
- Merlo A., Gabrielson E., Mabry M., Vollmer R., Baylin S. B., Sidransky D. Homozygous deletion on chromosome 9p and loss of heterozygosity on 9q, 6p, and 6q in primary human small cell lung cancer. Cancer Res. 1994 May 1;54(9):2322–2326. [PubMed] [Google Scholar]
- Munn K. E., Walker R. A., Menasce L., Varley J. M. Allelic imbalance in the region of the BRCA1 gene in ductal carcinoma in situ of the breast. Br J Cancer. 1996 Mar;73(5):636–639. doi: 10.1038/bjc.1996.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn K. E., Walker R. A., Varley J. M. Frequent alterations of chromosome 1 in ductal carcinoma in situ of the breast. Oncogene. 1995 Apr 20;10(8):1653–1657. [PubMed] [Google Scholar]
- Neville E. M., Stewart M., Myskow M., Donnelly R. J., Field J. K. Loss of heterozygosity at 9p23 defines a novel locus in non-small cell lung cancer. Oncogene. 1995 Aug 3;11(3):581–585. [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Olopade O. I., Buchhagen D. L., Malik K., Sherman J., Nobori T., Bader S., Nau M. M., Gazdar A. F., Minna J. D., Diaz M. O. Homozygous loss of the interferon genes defines the critical region on 9p that is deleted in lung cancers. Cancer Res. 1993 May 15;53(10 Suppl):2410–2415. [PubMed] [Google Scholar]
- Orlow I., Lianes P., Lacombe L., Dalbagni G., Reuter V. E., Cordon-Cardo C. Chromosome 9 allelic losses and microsatellite alterations in human bladder tumors. Cancer Res. 1994 Jun 1;54(11):2848–2851. [PubMed] [Google Scholar]
- Ottesen G. L., Graversen H. P., Blichert-Toft M., Zedeler K., Andersen J. A. Ductal carcinoma in situ of the female breast. Short-term results of a prospective nationwide study. The Danish Breast Cancer Cooperative Group. Am J Surg Pathol. 1992 Dec;16(12):1183–1196. doi: 10.1097/00000478-199212000-00005. [DOI] [PubMed] [Google Scholar]
- Radford D. M., Fair K. L., Phillips N. J., Ritter J. H., Steinbrueck T., Holt M. S., Donis-Keller H. Allelotyping of ductal carcinoma in situ of the breast: deletion of loci on 8p, 13q, 16q, 17p and 17q. Cancer Res. 1995 Aug 1;55(15):3399–3405. [PubMed] [Google Scholar]
- Ruppert J. M., Tokino K., Sidransky D. Evidence for two bladder cancer suppressor loci on human chromosome 9. Cancer Res. 1993 Nov 1;53(21):5093–5095. [PubMed] [Google Scholar]
- Schwartz G. F., Finkel G. C., Garcia J. C., Patchefsky A. S. Subclinical ductal carcinoma in situ of the breast. Treatment by local excision and surveillance alone. Cancer. 1992 Nov 15;70(10):2468–2474. doi: 10.1002/1097-0142(19921115)70:10<2468::aid-cncr2820701013>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Serrano M., Lee H., Chin L., Cordon-Cardo C., Beach D., DePinho R. A. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996 Apr 5;85(1):27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- Sonoda Y., Yoshimoto T., Sekiya T. Homozygous deletion of the MTS1/p16 and MTS2/p15 genes and amplification of the CDK4 gene in glioma. Oncogene. 1995 Nov 16;11(10):2145–2149. [PubMed] [Google Scholar]
- Stratton M. R., Wooster R. Hereditary predisposition to breast cancer. Curr Opin Genet Dev. 1996 Feb;6(1):93–97. doi: 10.1016/s0959-437x(96)90017-9. [DOI] [PubMed] [Google Scholar]
- Tarmin L., Yin J., Zhou X., Suzuki H., Jiang H. Y., Rhyu M. G., Abraham J. M., Krasna M. J., Cottrell J., Meltzer S. J. Frequent loss of heterozygosity on chromosome 9 in adenocarcinoma and squamous cell carcinoma of the esophagus. Cancer Res. 1994 Dec 1;54(23):6094–6096. [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Wilkie P. J., Krizman D. B., Weber J. L. Linkage map of human chromosome 9 microsatellite polymorphisms. Genomics. 1992 Mar;12(3):607–609. doi: 10.1016/0888-7543(92)90456-3. [DOI] [PubMed] [Google Scholar]
- Zhuang Z., Merino M. J., Chuaqui R., Liotta L. A., Emmert-Buck M. R. Identical allelic loss on chromosome 11q13 in microdissected in situ and invasive human breast cancer. Cancer Res. 1995 Feb 1;55(3):467–471. [PubMed] [Google Scholar]