Abstract
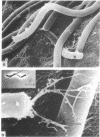
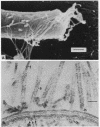
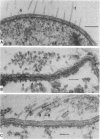
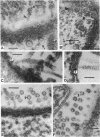
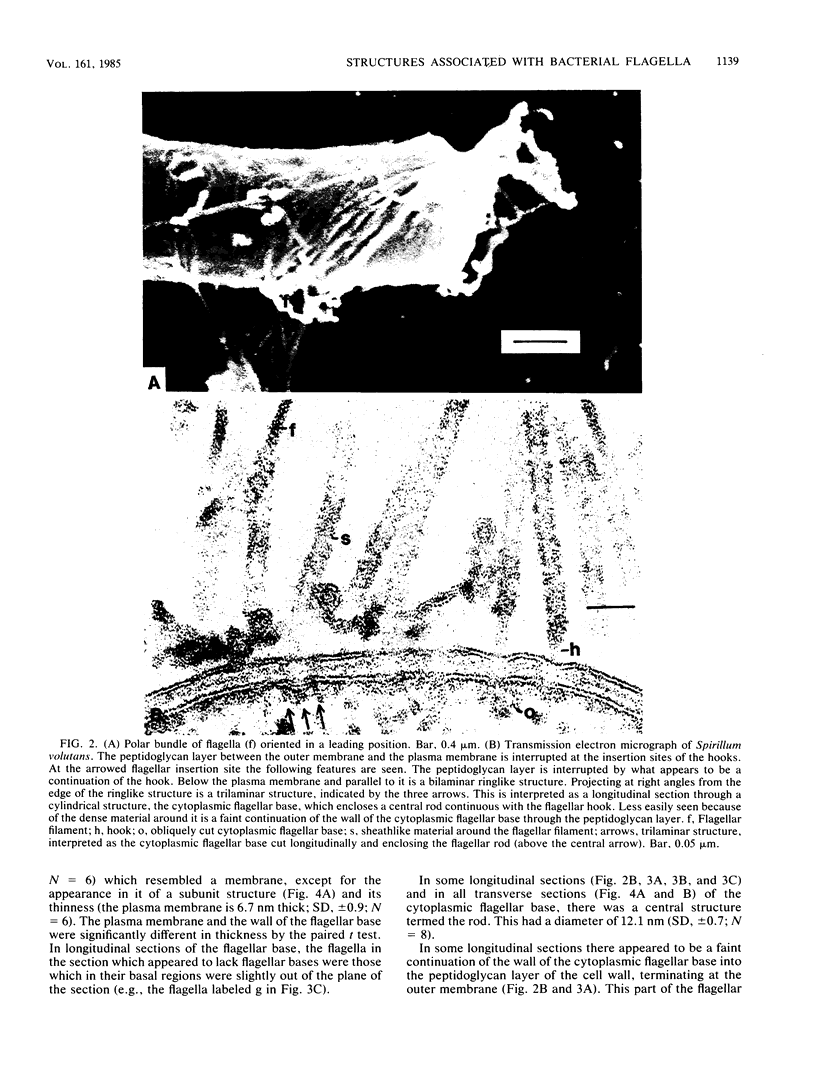
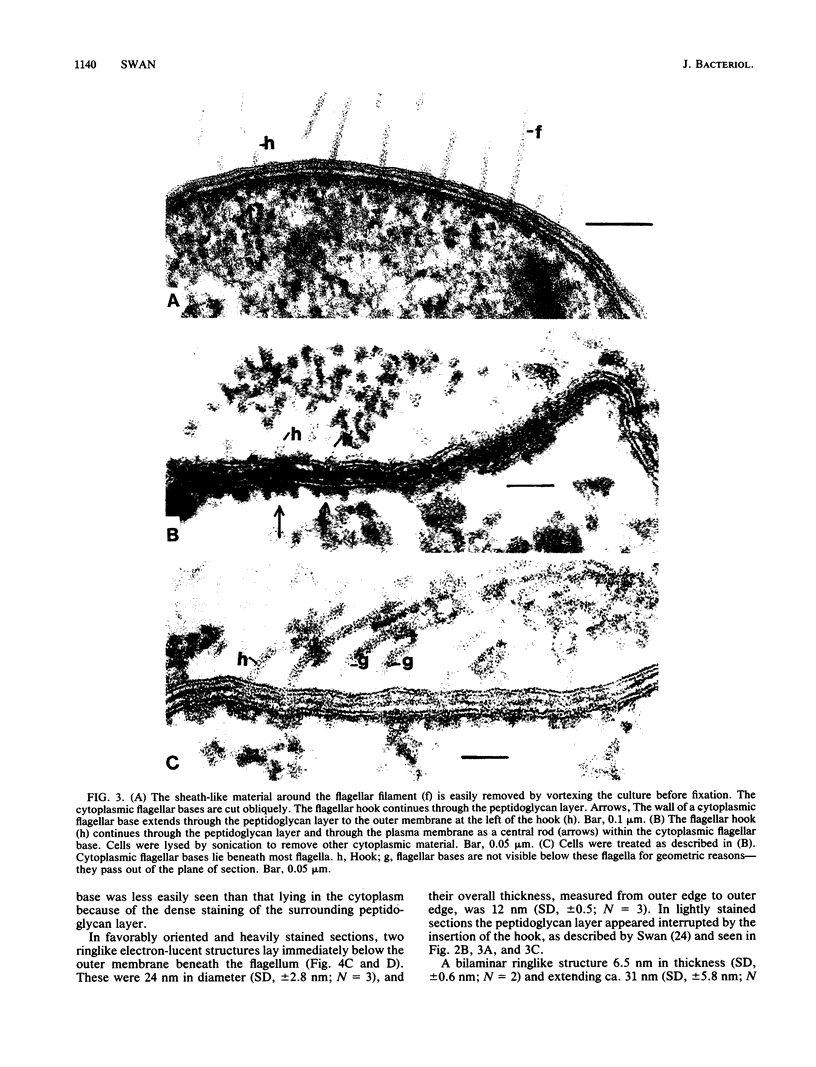
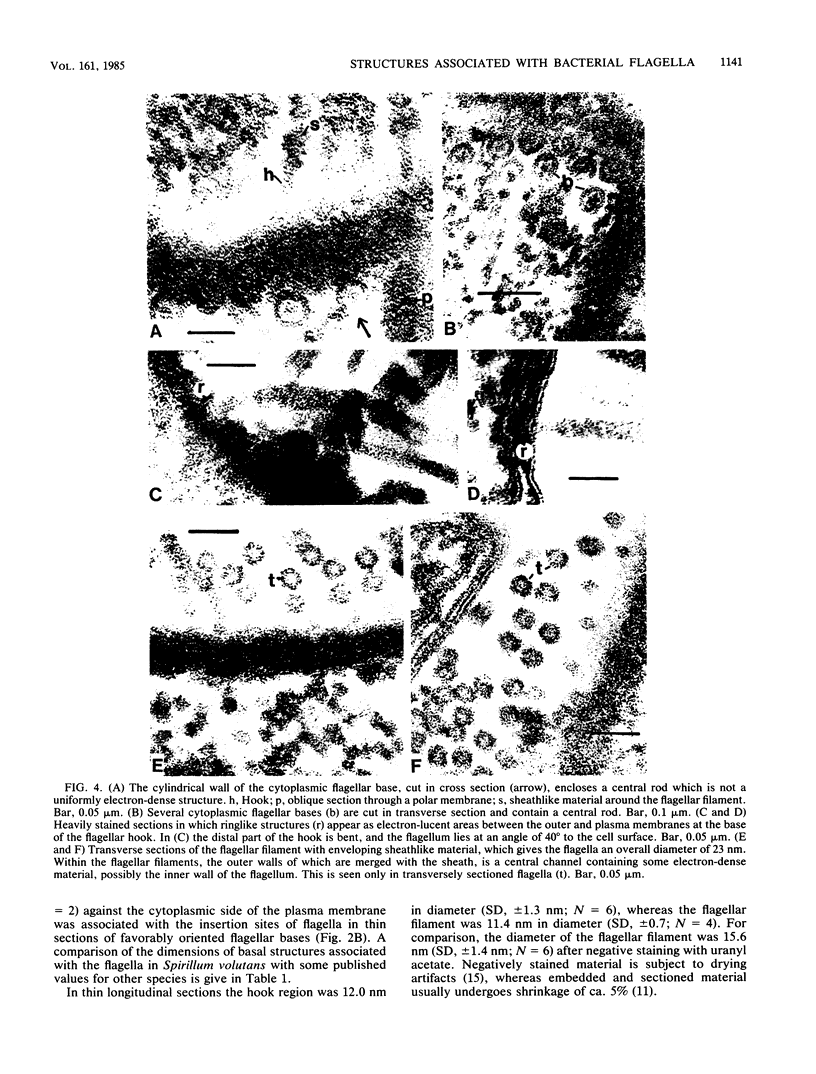
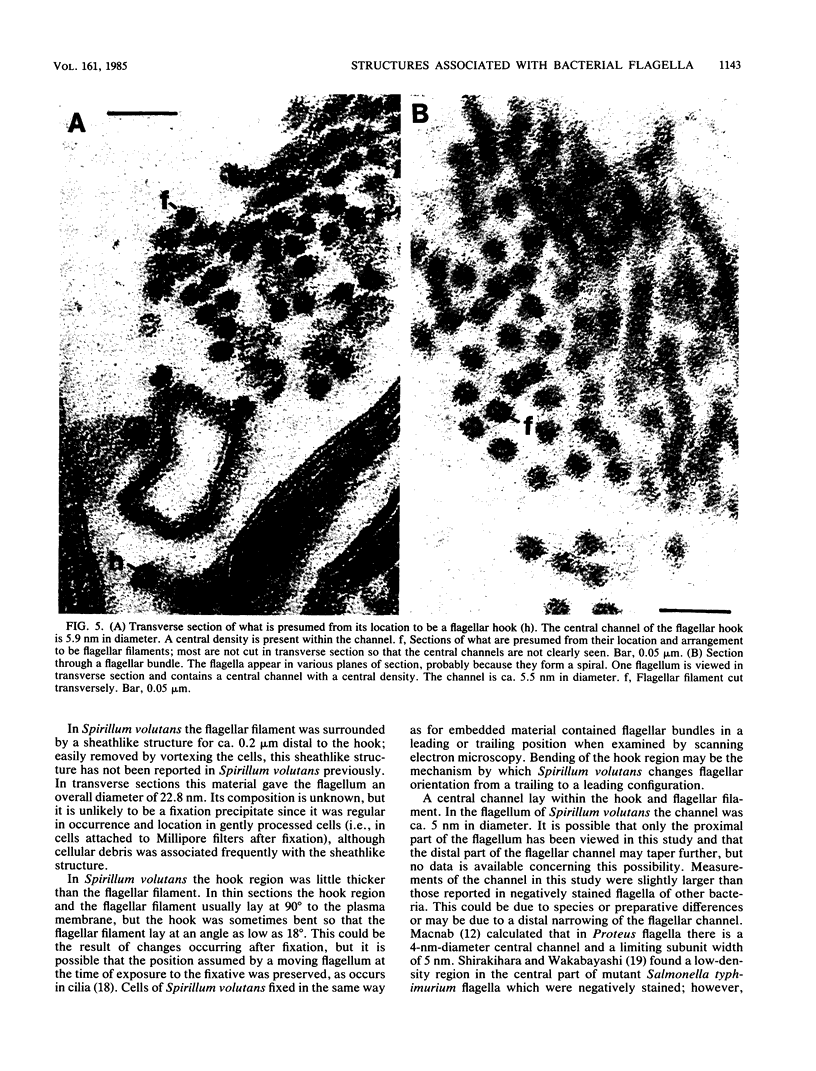
Electron microscopy of thin-sectioned Spirillum volutans (ATCC 19554) showed that at the insertion site of the flagellum there was a cylindrical structure with a diameter of ca. 36 nm which extended ca. 19 nm into the cytoplasm. This structure, termed a cytoplasmic flagellar base, enclosed a central rod which was continuous with the hook. There was a continuation of the flagellar base into the peptidoglycan layer, enclosing ringlike structures and the central rod. The flagellar hook and proximal part of the flagellar filament contained a central channel which was large enough to accommodate the flagellin subunit. The flagella of fixed cells may project perpendicularly from the outer membrane in a position corresponding to a trailing, swimming orientation or may bend almost parallel to the membrane in a leading orientation. Maximum bending occurred in the hook region, which may be the structure responsible for executing changes in swimming direction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg H. C., Anderson R. A. Bacteria swim by rotating their flagellar filaments. Nature. 1973 Oct 19;245(5425):380–382. doi: 10.1038/245380a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Bacterial behaviour. Nature. 1975 Apr 3;254(5499):389–392. doi: 10.1038/254389a0. [DOI] [PubMed] [Google Scholar]
- Chalcroft J. P., Bullivant S., Howard B. H. Ultrastructural studies on Selenomonas ruminantium from the sheep rumen. J Gen Microbiol. 1973 Nov;79(1):135–146. doi: 10.1099/00221287-79-1-135. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Murray R. G. Cell envelope associations of Aquaspirillum serpens flagella. J Bacteriol. 1978 Dec;136(3):1037–1049. doi: 10.1128/jb.136.3.1037-1049.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Attachment of flagellar basal bodies to the cell envelope: specific attachment to the outer, lipopolysaccharide membrane and the cyoplasmic membrane. J Bacteriol. 1971 Jan;105(1):396–407. doi: 10.1128/jb.105.1.396-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Purification of intact flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):376–383. doi: 10.1128/jb.105.1.376-383.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris F. G., Beveridge T. J., Marceau-Day M. L., Larson A. D. Structure and cell envelope associations of flagellar basal complexes of Vibrio cholerae and Campylobacter fetus. Can J Microbiol. 1984 Mar;30(3):322–333. doi: 10.1139/m84-048. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Kamiya R., Yamaguchi S. Excretion of flagellin by a short-flagella mutant of Salmonella typhimurium. J Bacteriol. 1983 Jan;153(1):506–510. doi: 10.1128/jb.153.1.506-510.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Walsh M. P., Ely B., Shapiro L. Flagellar hook and basal complex of Caulobacter crescentus. J Bacteriol. 1979 Jun;138(3):984–989. doi: 10.1128/jb.138.3.984-989.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Aizawa S. Bacterial motility and the bacterial flagellar motor. Annu Rev Biophys Bioeng. 1984;13:51–83. doi: 10.1146/annurev.bb.13.060184.000411. [DOI] [PubMed] [Google Scholar]
- Macnab R. M. Bacterial motility and chemotaxis: the molecular biology of a behavioral system. CRC Crit Rev Biochem. 1978;5(4):291–341. doi: 10.3109/10409237809177145. [DOI] [PubMed] [Google Scholar]
- Remsen C. C., Watson S. W., Waterbury J. B., Trüper H. G. Fine structure of Ectothiorhodospira mobilis Pelsh. J Bacteriol. 1968 Jun;95(6):2374–2392. doi: 10.1128/jb.95.6.2374-2392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATIR P. STUDIES ON CILIA. THE FIXATION OF THE METACHRONAL WAVE. J Cell Biol. 1963 Aug;18:345–365. doi: 10.1083/jcb.18.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T. Relation between thickness and interference colors of biological ultrathin section. J Electron Microsc (Tokyo) 1980;29(4):369–375. [PubMed] [Google Scholar]
- Shirakihara Y., Wakabayashi T. Three-dimensional image reconstruction of straight flagella from a mutant Salmonella typhimurium. J Mol Biol. 1979 Jul 5;131(3):485–507. doi: 10.1016/0022-2836(79)90004-4. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. I. Bacterial flagella. Annu Rev Microbiol. 1977;31:397–419. doi: 10.1146/annurev.mi.31.100177.002145. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Smith R. W., Koffler H. Bacterial flagella. Adv Microb Physiol. 1971;6:219–339. doi: 10.1016/s0065-2911(08)60070-3. [DOI] [PubMed] [Google Scholar]
- Swan M. A., Linck R. W., Ito S., Fawcett D. W. Structure and function of the undulating membrane in spermatozoan propulsion in the toad Bufo marinus. J Cell Biol. 1980 Jun;85(3):866–880. doi: 10.1083/jcb.85.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan M. A. Trailing flagella rotate faster than leading flagella in unipolar cells of Spirillum volutans. J Bacteriol. 1982 Apr;150(1):377–380. doi: 10.1128/jb.150.1.377-380.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Iterson W., Hoeniger J. F., Van Zanten E. N. Basal bodies of bacterial flagella in Proteus mirabilis. I. Electron microscopy of sectioned material. J Cell Biol. 1966 Dec;31(3):585–601. doi: 10.1083/jcb.31.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht T., DeRosier D. J., Aizawa S., Macnab R. M. Flagellar hook structures of Caulobacter and Salmonella and their relationship to filament structure. J Mol Biol. 1982 Nov 25;162(1):69–87. doi: 10.1016/0022-2836(82)90162-0. [DOI] [PubMed] [Google Scholar]