Abstract
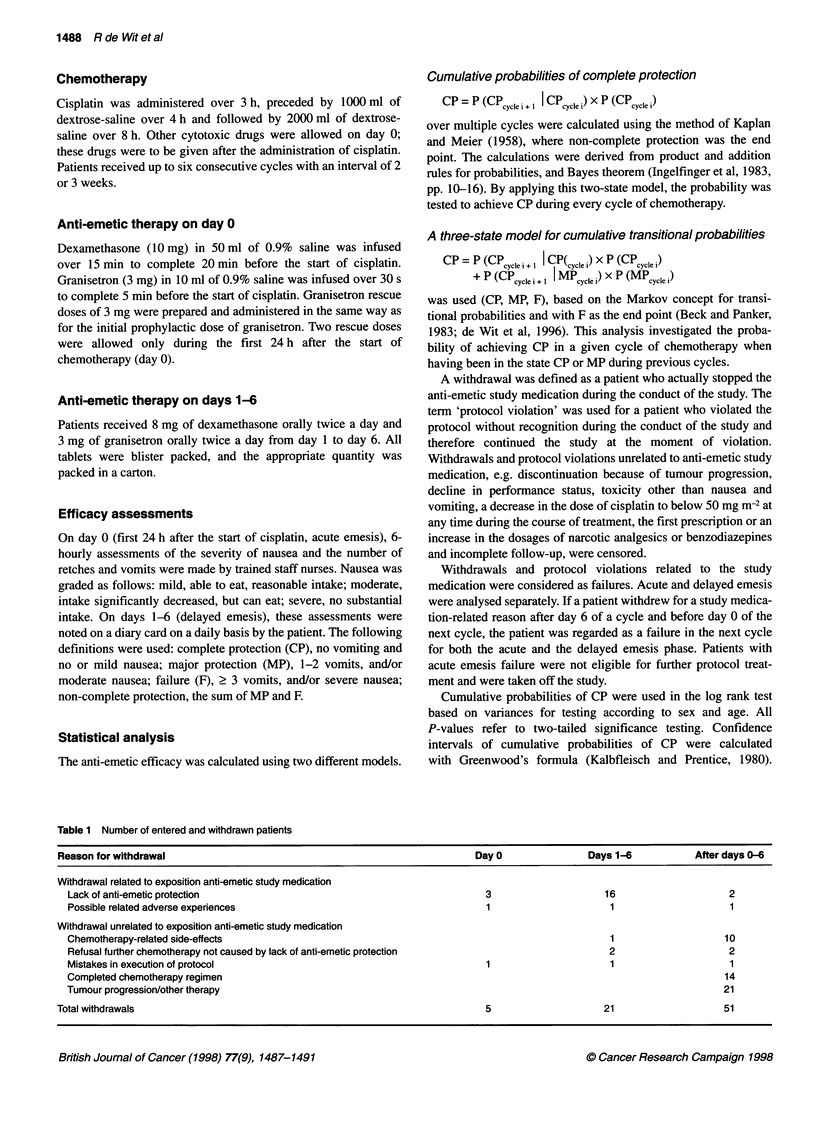
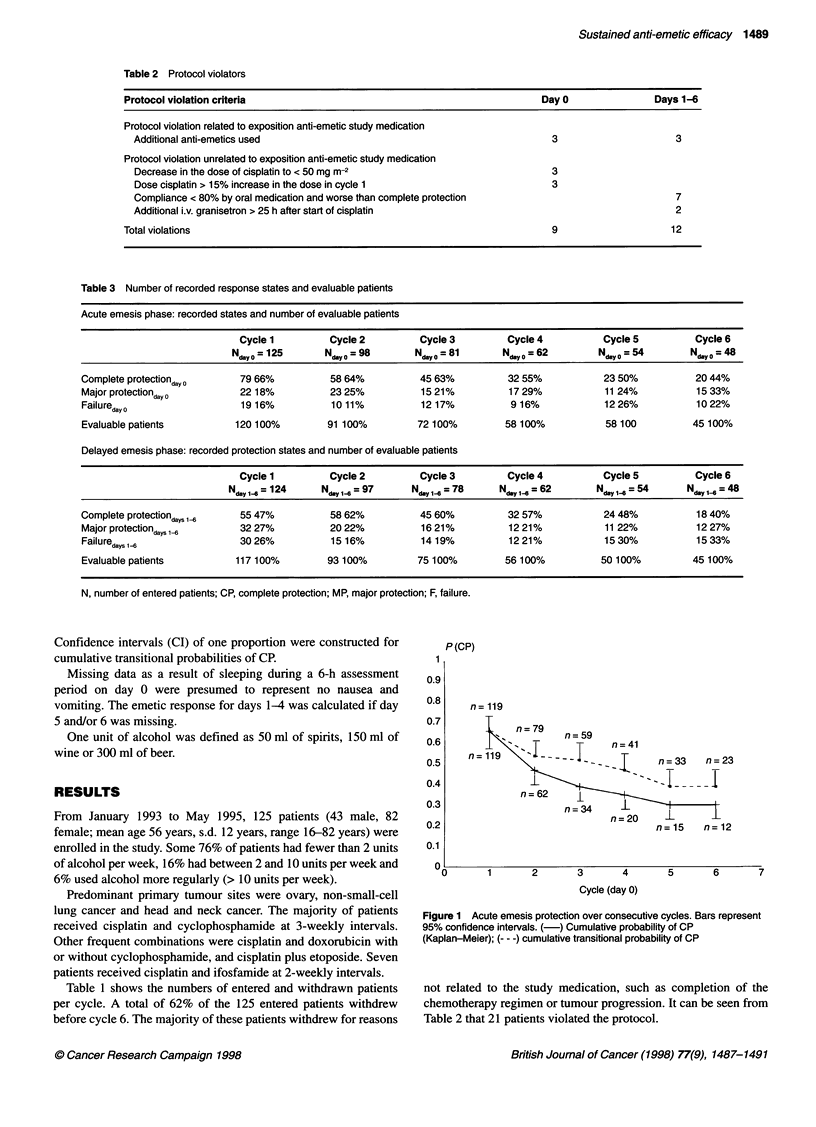
We have reported previously that the anti-emetic efficacy of single agent 5HT3 antagonists is not maintained when analysed with the measurement of cumulative probabilities. Presently, the most effective anti-emetic regimen is a combination of a 5HT3 antagonist plus dexamethasone. We, therefore, assessed the sustainment of efficacy of such a combination in 125 patients, scheduled to receive cisplatin > or = 70 mg m(-2) either alone or in combination with other cytotoxic drugs. Anti-emetic therapy was initiated with 10 mg of dexamethasone and 3 mg of granisetron intravenously, before cisplatin. On days 1-6, patients received 8 mg of dexamethasone and 1 mg of granisetron twice daily by oral administration. Protection was assessed during all cycles and calculated based on cumulative probability analyses using the method of Kaplan-Meier and a model for transitional probabilities. Irrespective of the type of analysis used, the anti-emetic efficacy of granisetron/dexamethasone decreased over cycles. The initial complete acute emesis protection rate of 66% decreased to 30% according to the method of Kaplan-Meier and to 39% using the model for transitional probabilities. For delayed emesis, the initial complete protection rate of 52% decreased to 21% (Kaplan-Meier) and to 43% (transitional probabilities). In addition, we observed that protection failure in the delayed emesis period adversely influenced the acute emesis protection in the next cycle. We conclude that the anti-emetic efficacy of a 5HT3 antagonist plus dexamethasone is not maintained over multiple cycles of highly emetogenic chemotherapy, and that the acute emesis protection is adversely influenced by protection failure in the delayed emesis phase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn M. J., Lee J. S., Lee K. H., Suh C., Choi S. S., Kim S. H. A randomized double-blind trial of ondansetron alone versus in combination with dexamethasone versus in combination with dexamethasone and lorazepam in the prevention of emesis due to cisplatin-based chemotherapy. Am J Clin Oncol. 1994 Apr;17(2):150–156. doi: 10.1097/00000421-199404000-00012. [DOI] [PubMed] [Google Scholar]
- Beck J. R., Pauker S. G. The Markov process in medical prognosis. Med Decis Making. 1983;3(4):419–458. doi: 10.1177/0272989X8300300403. [DOI] [PubMed] [Google Scholar]
- Coates A., Abraham S., Kaye S. B., Sowerbutts T., Frewin C., Fox R. M., Tattersall M. H. On the receiving end--patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983 Feb;19(2):203–208. doi: 10.1016/0277-5379(83)90418-2. [DOI] [PubMed] [Google Scholar]
- De Mulder P. H., Seynaeve C., Vermorken J. B., van Liessum P. A., Mols-Jevdevic S., Allman E. L., Beranek P., Verweij J. Ondansetron compared with high-dose metoclopramide in prophylaxis of acute and delayed cisplatin-induced nausea and vomiting. A multicenter, randomized, double-blind, crossover study. Ann Intern Med. 1990 Dec 1;113(11):834–840. doi: 10.7326/0003-4819-113-11-834. [DOI] [PubMed] [Google Scholar]
- Hainsworth J., Harvey W., Pendergrass K., Kasimis B., Oblon D., Monaghan G., Gandara D., Hesketh P., Khojasteh A., Harker G. A single-blind comparison of intravenous ondansetron, a selective serotonin antagonist, with intravenous metoclopramide in the prevention of nausea and vomiting associated with high-dose cisplatin chemotherapy. J Clin Oncol. 1991 May;9(5):721–728. doi: 10.1200/JCO.1991.9.5.721. [DOI] [PubMed] [Google Scholar]
- Hesketh P. J., Harvey W. H., Harker W. G., Beck T. M., Ryan T., Bricker L. J., Kish J. A., Murphy W. K., Hainsworth J. D., Haley B. A randomized, double-blind comparison of intravenous ondansetron alone and in combination with intravenous dexamethasone in the prevention of high-dose cisplatin-induced emesis. J Clin Oncol. 1994 Mar;12(3):596–600. doi: 10.1200/JCO.1994.12.3.596. [DOI] [PubMed] [Google Scholar]
- Marty M., Pouillart P., Scholl S., Droz J. P., Azab M., Brion N., Pujade-Lauraine E., Paule B., Paes D., Bons J. Comparison of the 5-hydroxytryptamine3 (serotonin) antagonist ondansetron (GR 38032F) with high-dose metoclopramide in the control of cisplatin-induced emesis. N Engl J Med. 1990 Mar 22;322(12):816–821. doi: 10.1056/NEJM199003223221205. [DOI] [PubMed] [Google Scholar]
- Roila F., Tonato M., Cognetti F., Cortesi E., Favalli G., Marangolo M., Amadori D., Bella M. A., Gramazio V., Donati D. Prevention of cisplatin-induced emesis: a double-blind multicenter randomized crossover study comparing ondansetron and ondansetron plus dexamethasone. J Clin Oncol. 1991 Apr;9(4):675–678. doi: 10.1200/JCO.1991.9.4.675. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Newlands E. S., Rustin G. J., Begent R. H., Howells N., McQuade B., Bagshawe K. D. Comparison of ondansetron and ondansetron plus dexamethasone as antiemetic prophylaxis during cisplatin-containing chemotherapy. Lancet. 1991 Aug 24;338(8765):487–490. doi: 10.1016/0140-6736(91)90555-4. [DOI] [PubMed] [Google Scholar]
- de Wit R., Schmitz P. I., Verweij J., de Boer-Dennert M., de Mulder P. H., Planting A. S., van der Burg M. E., Stoter G. Analysis of cumulative probabilities shows that the efficacy of 5HT3 antagonist prophylaxis is not maintained. J Clin Oncol. 1996 Feb;14(2):644–651. doi: 10.1200/JCO.1996.14.2.644. [DOI] [PubMed] [Google Scholar]


