Abstract
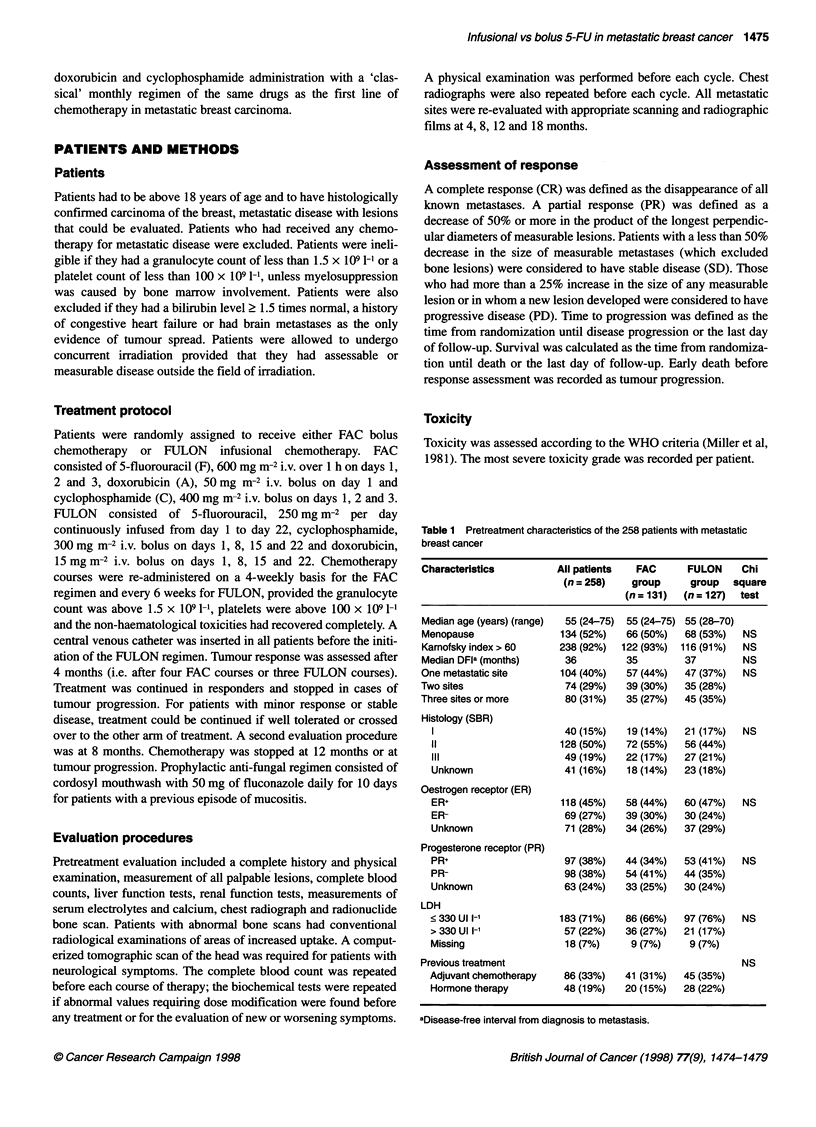
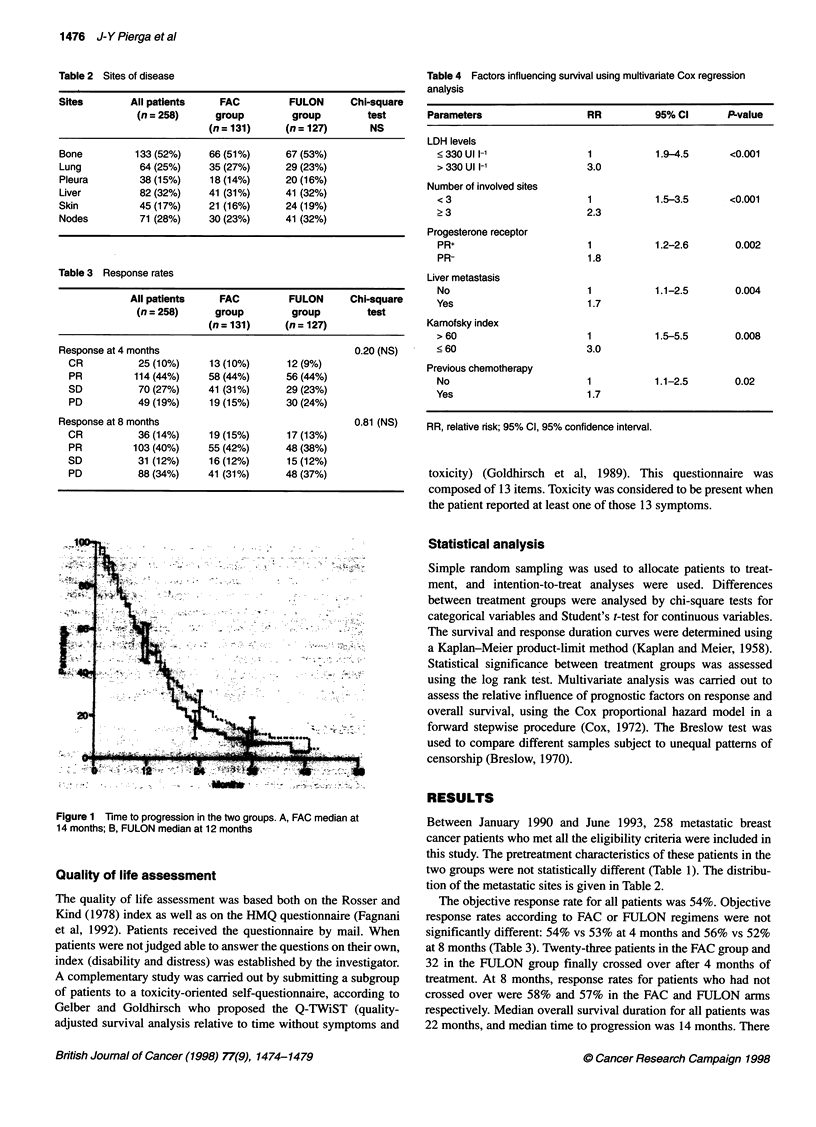
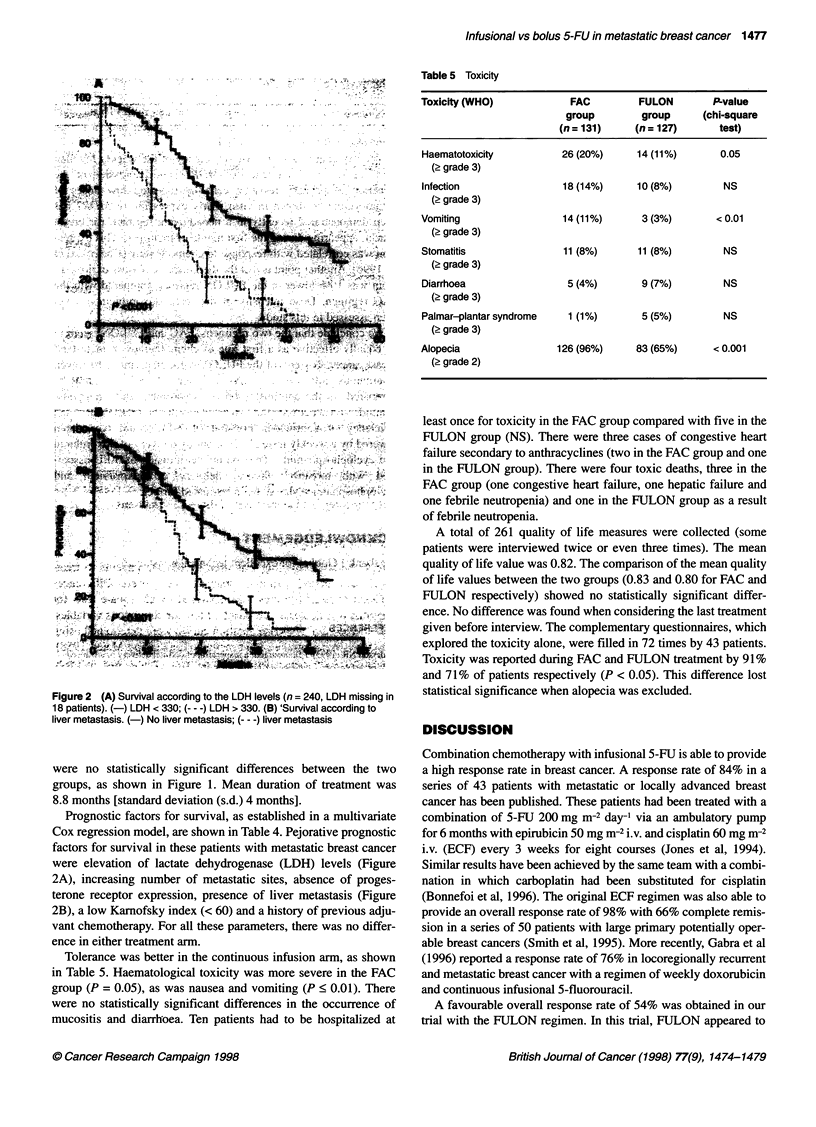
Infusional 5-fluorouracil in advanced breast cancer has been associated with improved clinical response rates when compared with conventional bolus therapy. As a first line of chemotherapy in proven metastatic breast carcinoma, 258 women were randomly assigned to receive FAC consisting of 5-fluorouracil (F) 600 mg m(-2) intravenously (i.v.) over 1 h on days 1, 2 and 3, doxorubicin (A) 50 mg m(-2) i.v. bolus on day 1 and cyclophosphamide (C), 400 mg m(-2) i.v. bolus on days 1, 2 and 3 or 'FULON' consisting of 5-fluorouracil 250 mg m(-2) day(-1) continuously infused from day 1 to day 22, doxorubicin 15 mg m(-2) i.v. bolus on days 1, 8, 15 and 22 and cyclophosphamide 300 mg m(-2) i.v. bolus on days 1, 8, 15 and 22. Chemotherapy courses were administered 4-weekly for the bolus regimen and 6-weekly for FULON. Pretreatment characteristics were identical between the two groups. Response rates were 54% in the FAC arm and 53% in the FULON arm. Time to progression was 14 months in the FAC arm and 12 months in the FULON arm. Differences were not statistically significant. Median overall survival duration for all patients was 22 months. Haematological toxicity was more severe in the bolus-treated group (P = 0.05), as were nausea and vomiting (P < or = 0.01). We conclude that the two regimens appeared equally effective but have different toxicities.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin R. S., Wiernik P. H., Bachur N. R. Adriamycin chemotherapy--efficacy, safety, and pharmacologic basis of an intermittent single high-dosage schedule. Cancer. 1974 Jan;33(1):19–27. doi: 10.1002/1097-0142(197401)33:1<19::aid-cncr2820330107>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Blomqvist C., Elomaa I., Rissanen P., Hietanen P., Nevasaari K., Helle L. FEC (5-fluorouracil-epirubicin-cyclophosphamide) monthly versus FEC weekly in metastatic breast cancer. First results of a randomized trial. Acta Oncol. 1992;31(2):231–236. doi: 10.3109/02841869209088908. [DOI] [PubMed] [Google Scholar]
- Bonnefoi H., Smith I. E., O'Brien M. E., Seymour M. T., Powles T. J., Allum W. H., Ebbs S., Baum M. Phase II study of continuous infusional 5-fluorouracil with epirubicin and carboplatin (instead of cisplatin) in patients with metastatic/locally advanced breast cancer (infusional ECarboF): a very active and well-tolerated outpatient regimen. Br J Cancer. 1996 Feb;73(3):391–396. doi: 10.1038/bjc.1996.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneterre J., Mercier M. Response to chemotherapy after relapse in patients with or without previous adjuvant chemotherapy for breast cancer. The French Epirubicin Study Group. Cancer Treat Rev. 1993 Apr;19 (Suppl B):21–30. doi: 10.1016/0305-7372(93)90004-b. [DOI] [PubMed] [Google Scholar]
- Carter S. K. Integration of chemotherapy into combined modality treatment of solid tumors VII. Adenocarcinoma of the breast. Cancer Treat Rev. 1976 Sep;3(3):141–174. doi: 10.1016/s0305-7372(76)80020-5. [DOI] [PubMed] [Google Scholar]
- Chevillard S., Pouillart P., Beldjord C., Asselain B., Beuzeboc P., Magdelénat H., Vielh P. Sequential assessment of multidrug resistance phenotype and measurement of S-phase fraction as predictive markers of breast cancer response to neoadjuvant chemotherapy. Cancer. 1996 Jan 15;77(2):292–300. doi: 10.1002/(SICI)1097-0142(19960115)77:2<292::AID-CNCR11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Chlebowski R. T., Weiner J. M., Luce J., Hestorff R., Lang J. E., Reynolds R., Godfrey T., Ryden V. M., Bateman J. R. Significance of relapse after adjuvant treatment with combination chemotherapy or 5-fluorouracil alone in high-risk breast cancer. A Western Cancer Study Group Project. Cancer Res. 1981 Nov;41(11 Pt 1):4399–4403. [PubMed] [Google Scholar]
- Fraile R. J., Baker L. H., Buroker T. R., Horwitz J., Vaitkevicius V. K. Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res. 1980 Jul;40(7):2223–2228. [PubMed] [Google Scholar]
- Gabra H., Cameron D. A., Lee L. E., Mackay J., Leonard R. C. Weekly doxorubicin and continuous infusional 5-fluorouracil for advanced breast cancer. Br J Cancer. 1996 Dec;74(12):2008–2012. doi: 10.1038/bjc.1996.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhirsch A., Gelber R. D., Simes R. J., Glasziou P., Coates A. S. Costs and benefits of adjuvant therapy in breast cancer: a quality-adjusted survival analysis. J Clin Oncol. 1989 Jan;7(1):36–44. doi: 10.1200/JCO.1989.7.1.36. [DOI] [PubMed] [Google Scholar]
- Hansen R. M. 5-Fluorouracil by protracted venous infusion: a review of recent clinical studies. Cancer Invest. 1991;9(6):637–642. doi: 10.3109/07357909109039875. [DOI] [PubMed] [Google Scholar]
- Hansen R., Quebbeman E., Beatty P., Ritch P., Anderson T., Jenkins D., Frick J., Ausman R. Continuous 5-fluorouracil infusion in refractory carcinoma of the breast. Breast Cancer Res Treat. 1987 Nov;10(2):145–149. doi: 10.1007/BF01810577. [DOI] [PubMed] [Google Scholar]
- Hortobagyi G. N., Smith T. L., Legha S. S., Swenerton K. D., Gehan E. A., Yap H. Y., Buzdar A. U., Blumenschein G. R. Multivariate analysis of prognostic factors in metastatic breast cancer. J Clin Oncol. 1983 Dec;1(12):776–786. doi: 10.1200/JCO.1983.1.12.776. [DOI] [PubMed] [Google Scholar]
- Houston S. J., Richards M. A., Bentley A. E., Smith P., Rubens R. D. The influence of adjuvant chemotherapy on outcome after relapse for patients with breast cancer. Eur J Cancer. 1993;29A(11):1513–1518. doi: 10.1016/0959-8049(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Hryniuk W. M., Levine M. N., Levin L. Analysis of dose intensity for chemotherapy in early (stage II) and advanced breast cancer. NCI Monogr. 1986;(1):87–94. [PubMed] [Google Scholar]
- Huan S., Pazdur R., Singhakowinta A., Samal B., Vaitkevicius V. K. Low-dose continuous infusion 5-fluorouracil. Evaluation in advanced breast carcinoma. Cancer. 1989 Feb 1;63(3):419–422. doi: 10.1002/1097-0142(19890201)63:3<419::aid-cncr2820630303>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Smith I. E., O'Brien M. E., Talbot D., Walsh G., Ramage F., Robertshaw H., Ashley S. Phase II study of continuous infusion fluorouracil with epirubicin and cisplatin in patients with metastatic and locally advanced breast cancer: an active new regimen. J Clin Oncol. 1994 Jun;12(6):1259–1265. doi: 10.1200/JCO.1994.12.6.1259. [DOI] [PubMed] [Google Scholar]
- Jouve M., Palangie T., Belli L., Dorval T., Garcia-Giralt E., Beuzeboc P., Scholl S., Mosseri V., Livartowski A., Vedrenne J. Cancer du sein métastase. Chimiothérapie par adriamycine, vindésine, cyclophosphamide et 5 FU. Intérêt de la perfusion continue de 5 FU. Résultats d'un essai contrôlé. Bull Cancer. 1989;76(6):643–652. [PubMed] [Google Scholar]
- Lokich J., Anderson N. Infusional cancer chemotherapy: historical evolution and future development at the Cancer Center of Boston. Cancer Invest. 1995;13(2):202–226. doi: 10.3109/07357909509011691. [DOI] [PubMed] [Google Scholar]
- Lokich J., Bothe A., Fine N., Perri J. Phase I study of protracted venous infusion of 5-fluorouracil. Cancer. 1981 Dec 15;48(12):2565–2568. doi: 10.1002/1097-0142(19811215)48:12<2565::aid-cncr2820481204>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Raymond E., Palangie T., Jouve M., Asselain B., Dieras V., Beuzeboc P., Dorval T., Garcia-Giralt E., Livartowski A., Scholl S. Protracted continuous infusion of 5-fluorouracil in combination with doxorubicin, vincristine, and oral cyclophosphamide in advanced breast cancer. Cancer Invest. 1996;14(2):91–97. doi: 10.3109/07357909609018882. [DOI] [PubMed] [Google Scholar]
- Ross M. B., Buzdar A. U., Smith T. L., Eckles N., Hortobagyi G. N., Blumenschein G. R., Freireich E. J., Gehan E. A. Improved survival of patients with metastatic breast cancer receiving combination chemotherapy. Cancer. 1985 Jan 15;55(2):341–346. doi: 10.1002/1097-0142(19850115)55:2<341::aid-cncr2820550206>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Rosser R., Kind P. A scale of valuations of states of illness: is there a social consensus? Int J Epidemiol. 1978 Dec;7(4):347–358. doi: 10.1093/ije/7.4.347. [DOI] [PubMed] [Google Scholar]
- Rubens R. D. Effect of adjuvant systemic therapy on response to treatment after relapse. Cancer Treat Rev. 1993 Apr;19 (Suppl B):3–10. doi: 10.1016/0305-7372(93)90002-9. [DOI] [PubMed] [Google Scholar]
- Scheithauer W., Zielinksi C., Ludwig H. Weekly low dose doxorubicin monotherapy in metastatic breast cancer resistant to previous hormonal and cytostatic treatment. Breast Cancer Res Treat. 1985;6(1):89–93. doi: 10.1007/BF01806014. [DOI] [PubMed] [Google Scholar]
- Smith I. E., Walsh G., Jones A., Prendiville J., Johnston S., Gusterson B., Ramage F., Robertshaw H., Sacks N., Ebbs S. High complete remission rates with primary neoadjuvant infusional chemotherapy for large early breast cancer. J Clin Oncol. 1995 Feb;13(2):424–429. doi: 10.1200/JCO.1995.13.2.424. [DOI] [PubMed] [Google Scholar]
- Twelves C. J., O'Reilly S. M., Coleman R. E., Richards M. A., Rubens R. D. Weekly epirubicin for breast cancer with liver metastases and abnormal liver biochemistry. Br J Cancer. 1989 Dec;60(6):938–941. doi: 10.1038/bjc.1989.394. [DOI] [PMC free article] [PubMed] [Google Scholar]


