Abstract
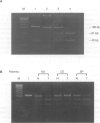
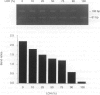
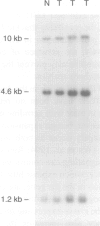
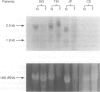
Expression of Fas, an apoptosis-inducing receptor, in colonic epithelium is progressively reduced during malignant transformation. We have examined the human Fas gene for loss of heterozygosity (LOH) and gross rearrangements in colon tumours and matched normal mucosa. Polymerase chain reaction (PCR) primers were designed to span a DraI restriction fragment length polymorphic site in the gene. Heterozygosity was detected in normal DNA samples by PCR amplification of the polymorphic site and restriction enzyme digestion. Thirty-eight of 88 patients (43%) with colon carcinomas were informative for the assay, and LOH was detected in 6 of the 38 (16%) corresponding tumours. Tumours from three patients with LOH did not express detectable Fas mRNA, and Fas expression was reduced or absent in 7 of 11 tumours from informative patients without LOH. Southern blotting of tumour DNA samples was used to detect rearrangement of the Fas gene, but no altered hybridization patterns were observed in 64 tumours analysed. These findings indicate that disruption of the Fas gene is not primarily responsible for the loss of Fas protein expression reported in colon cancer. We have also shown that loss of Fas gene transcription is common in these tumours, which may be due to epigenetic gene silencing.
Full text
PDF

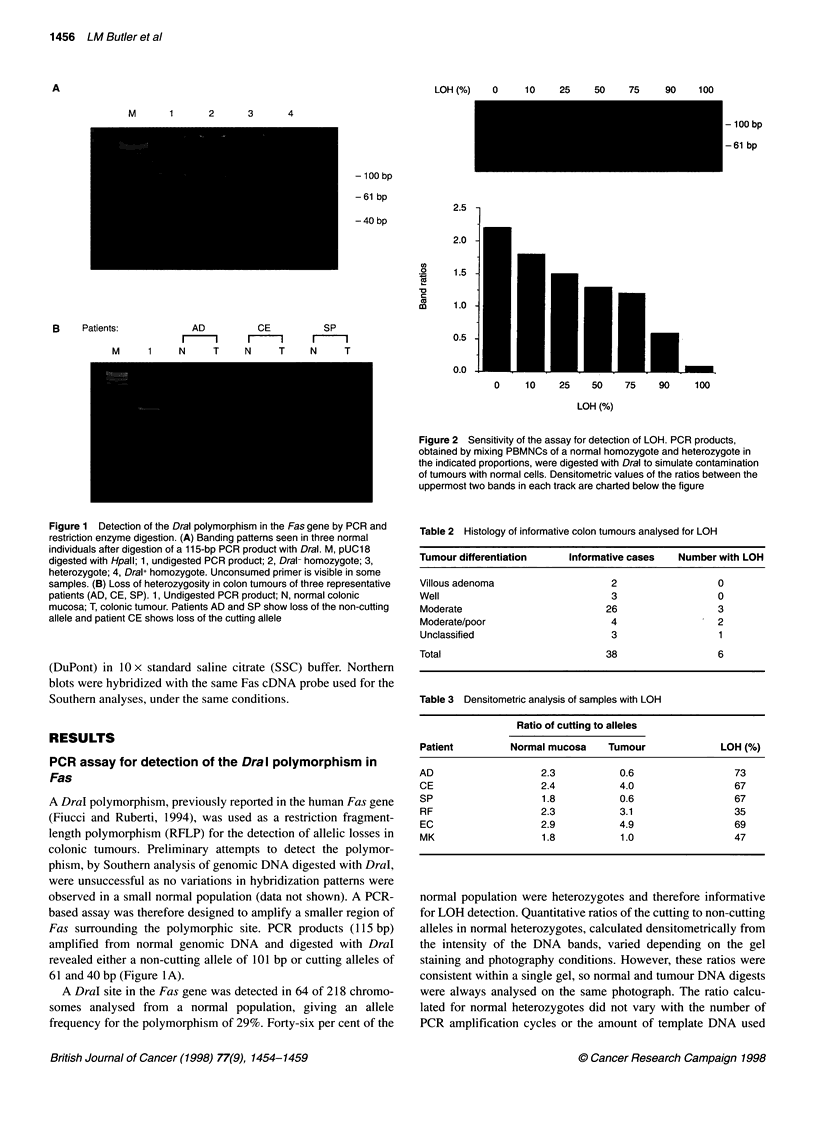
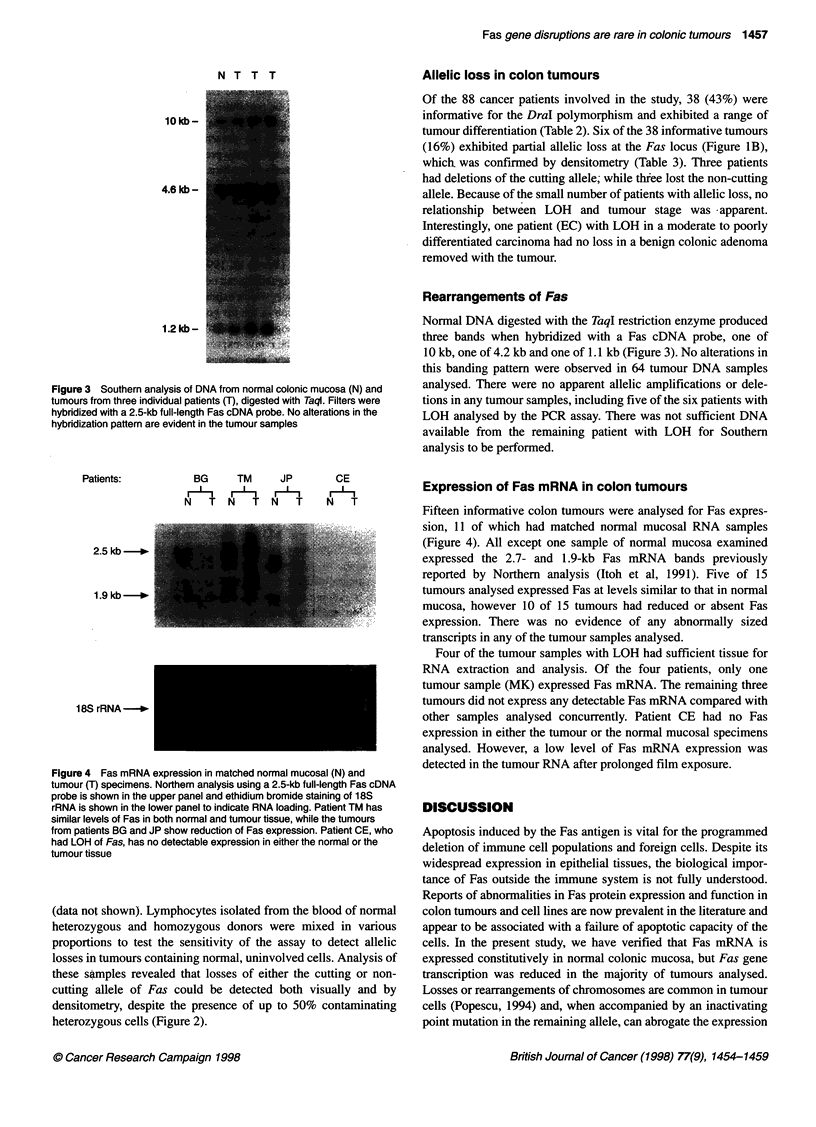


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abreu-Martin M. T., Vidrich A., Lynch D. H., Targan S. R. Divergent induction of apoptosis and IL-8 secretion in HT-29 cells in response to TNF-alpha and ligation of Fas antigen. J Immunol. 1995 Nov 1;155(9):4147–4154. [PubMed] [Google Scholar]
- Adachi M., Suematsu S., Kondo T., Ogasawara J., Tanaka T., Yoshida N., Nagata S. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet. 1995 Nov;11(3):294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- Behrmann I., Walczak H., Krammer P. H. Structure of the human APO-1 gene. Eur J Immunol. 1994 Dec;24(12):3057–3062. doi: 10.1002/eji.1830241221. [DOI] [PubMed] [Google Scholar]
- Cascino I., Papoff G., De Maria R., Testi R., Ruberti G. Fas/Apo-1 (CD95) receptor lacking the intracytoplasmic signaling domain protects tumor cells from Fas-mediated apoptosis. J Immunol. 1996 Jan 1;156(1):13–17. [PubMed] [Google Scholar]
- Cheng J., Liu C., Koopman W. J., Mountz J. D. Characterization of human Fas gene. Exon/intron organization and promoter region. J Immunol. 1995 Feb 1;154(3):1239–1245. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- De Maria R., Boirivant M., Cifone M. G., Roncaioli P., Hahne M., Tschopp J., Pallone F., Santoni A., Testi R. Functional expression of Fas and Fas ligand on human gut lamina propria T lymphocytes. A potential role for the acidic sphingomyelinase pathway in normal immunoregulation. J Clin Invest. 1996 Jan 15;97(2):316–322. doi: 10.1172/JCI118418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhein J., Walczak H., Bäumler C., Debatin K. M., Krammer P. H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995 Feb 2;373(6513):438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Drappa J., Vaishnaw A. K., Sullivan K. E., Chu J. L., Elkon K. B. Fas gene mutations in the Canale-Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N Engl J Med. 1996 Nov 28;335(22):1643–1649. doi: 10.1056/NEJM199611283352204. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fiucci G., Ruberti G. Detection of polymorphisms within the Fas cDNA gene sequence by GC-clamp denaturing gradient gel electrophoresis. Immunogenetics. 1994;39(6):437–439. doi: 10.1007/BF00176163. [DOI] [PubMed] [Google Scholar]
- Garewal H., Bernstein H., Bernstein C., Sampliner R., Payne C. Reduced bile acid-induced apoptosis in "normal" colorectal mucosa: a potential biological marker for cancer risk. Cancer Res. 1996 Apr 1;56(7):1480–1483. [PubMed] [Google Scholar]
- Hakuno N., Koji T., Yano T., Kobayashi N., Tsutsumi O., Taketani Y., Nakane P. K. Fas/APO-1/CD95 system as a mediator of granulosa cell apoptosis in ovarian follicle atresia. Endocrinology. 1996 May;137(5):1938–1948. doi: 10.1210/endo.137.5.8612534. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Coates P. J., Ansari B., Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994 Dec;107(Pt 12):3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- Inazawa J., Itoh N., Abe T., Nagata S. Assignment of the human Fas antigen gene (Fas) to 10q24.1. Genomics. 1992 Nov;14(3):821–822. doi: 10.1016/s0888-7543(05)80200-9. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Winterford C. M., Harmon B. V. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994 Apr 15;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Koetsier P. A., Schorr J., Doerfler W. A rapid optimized protocol for downward alkaline Southern blotting of DNA. Biotechniques. 1993 Aug;15(2):260–262. [PubMed] [Google Scholar]
- Leithäuser F., Dhein J., Mechtersheimer G., Koretz K., Brüderlein S., Henne C., Schmidt A., Debatin K. M., Krammer P. H., Möller P. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993 Oct;69(4):415–429. [PubMed] [Google Scholar]
- Lichter P., Walczak H., Weitz S., Behrmann I., Krammer P. H. The human APO-1 (APT) antigen maps to 10q23, a region that is syntenic with mouse chromosome 19. Genomics. 1992 Sep;14(1):179–180. doi: 10.1016/s0888-7543(05)80302-7. [DOI] [PubMed] [Google Scholar]
- Muleris M., Salmon R. J., Dutrillaux B. Cytogenetics of colorectal adenocarcinomas. Cancer Genet Cytogenet. 1990 Jun;46(2):143–156. doi: 10.1016/0165-4608(90)90100-o. [DOI] [PubMed] [Google Scholar]
- Möller P., Koretz K., Leithäuser F., Brüderlein S., Henne C., Quentmeier A., Krammer P. H. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer. 1994 May 1;57(3):371–377. doi: 10.1002/ijc.2910570314. [DOI] [PubMed] [Google Scholar]
- O'Connell J., O'Sullivan G. C., Collins J. K., Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996 Sep 1;184(3):1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Schaub L. B., Meterissian S., Ford R. J. Fas/APO-1 expression and function on malignant cells of hematologic and nonhematologic origin. J Immunother Emphasis Tumor Immunol. 1993 Oct;14(3):234–241. doi: 10.1097/00002371-199310000-00011. [DOI] [PubMed] [Google Scholar]
- Owen-Schaub L. B., Radinsky R., Kruzel E., Berry K., Yonehara S. Anti-Fas on nonhematopoietic tumors: levels of Fas/APO-1 and bcl-2 are not predictive of biological responsiveness. Cancer Res. 1994 Mar 15;54(6):1580–1586. [PubMed] [Google Scholar]
- Owen-Schaub L. B., Zhang W., Cusack J. C., Angelo L. S., Santee S. M., Fujiwara T., Roth J. A., Deisseroth A. B., Zhang W. W., Kruzel E. Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol. 1995 Jun;15(6):3032–3040. doi: 10.1128/mcb.15.6.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C. M., Bernstein H., Bernstein C., Garewal H. Role of apoptosis in biology and pathology: resistance to apoptosis in colon carcinogenesis. Ultrastruct Pathol. 1995 Jul-Aug;19(4):221–248. doi: 10.3109/01913129509064227. [DOI] [PubMed] [Google Scholar]
- Peng S. L., Robert M. E., Hayday A. C., Craft J. A tumor-suppressor function for Fas (CD95) revealed in T cell-deficient mice. J Exp Med. 1996 Sep 1;184(3):1149–1154. doi: 10.1084/jem.184.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu N. C. Chromosome fragility and instability in human cancer. Crit Rev Oncog. 1994;5(2-3):121–140. doi: 10.1615/critrevoncog.v5.i2-3.20. [DOI] [PubMed] [Google Scholar]
- Rieux-Laucat F., Le Deist F., Hivroz C., Roberts I. A., Debatin K. M., Fischer A., de Villartay J. P. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995 Jun 2;268(5215):1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- Rouvier E., Luciani M. F., Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med. 1993 Jan 1;177(1):195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott N., Martin I., Jack A. S., Dixon M. F., Quirke P. Genes mediating programmed cell death: an immunohistochemical study of bcl-2, c-myc and p53 expression in colorectal neoplasia. Clin Mol Pathol. 1996 Jun;49(3):M151–M158. doi: 10.1136/mp.49.3.m151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinicrope F. A., Ruan S. B., Cleary K. R., Stephens L. C., Lee J. J., Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995 Jan 15;55(2):237–241. [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Wu J., Zhou T., Zhang J., He J., Gause W. C., Mountz J. D. Correction of accelerated autoimmune disease by early replacement of the mutated lpr gene with the normal Fas apoptosis gene in the T cells of transgenic MRL-lpr/lpr mice. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2344–2348. doi: 10.1073/pnas.91.6.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerri L., Carbuccia N., Parc P., Birg F. Search for rearrangements and/or allelic loss of the fas/APO-1 gene in 101 human lymphomas. Am J Clin Pathol. 1995 Oct;104(4):424–430. doi: 10.1093/ajcp/104.4.424. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zörnig M., Grzeschiczek A., Kowalski M. B., Hartmann K. U., Möröy T. Loss of Fas/Apo-1 receptor accelerates lymphomagenesis in E mu L-MYC transgenic mice but not in animals infected with MoMuLV. Oncogene. 1995 Jun 15;10(12):2397–2401. [PubMed] [Google Scholar]