Abstract
Glucose is an essential signaling molecule that controls plant development and gene expression through largely unknown mechanisms. To initiate the dissection of the glucose signal transduction pathway in plants by using a genetic approach, we have identified an Arabidopsis mutant, gin1 (glucose-insensitive), in which glucose repression of cotyledon greening and expansion, shoot development, floral transition, and gene expression is impaired. Genetic analysis indicates that GIN1 acts downstream of the sensor hexokinase in the glucose signaling pathway. Surprisingly, gin1 insensitivity to glucose repression of cotyledon and shoot development is phenocopied by ethylene precursor treatment of wild-type plants or by constitutive ethylene biosynthesis and constitutive ethylene signaling mutants. In contrast, the ethylene insensitive mutant etr1-1 exhibits glucose hypersensitivity. Epistasis analysis places GIN1 downstream of the ethylene receptor, ETR1, and defines a new branch of ethylene signaling pathway that is uncoupled from the triple response induced by ethylene. The isolation and characterization of gin1 reveal an unexpected convergence between the glucose and the ethylene signal transduction pathways. GIN1 may function to balance the control of plant development in response to metabolic and hormonal stimuli that act antagonistically.
Glucose has profound effects on gene expression, metabolism, and development in microorganisms, animals, and plants (1–9). Although the glucose signal transduction pathways are well characterized in unicellular microorganisms, relatively little is known about the molecular basis of glucose responses in multicellular eukaryotes. In higher plants, glucose has been implicated to be the primary sugar signal that controls many aspects of plant development, including germination, hypocotyl elongation, cotyledon greening and expansion, primary and lateral root growth, true leaf development, floral transition, and the onset of senescence. At the molecular level, the expression of a broad spectrum of genes is either repressed or induced by glucose (4–9). Recently, hexokinase (HXK), the enzyme that catalyzes the phosphorylation of hexose sugars at the first step of the glycolytic pathway, has been shown to be the glucose sensor in plants (9–12). Studies in transgenic Arabidopsis plants with elevated or reduced Arabidopsis thaliana HXK levels or with a heterologous yeast HXK provide supporting evidence that HXK is a bifunctional enzyme with catalytic and regulatory activities, and glucose signaling may be uncoupled from glucose metabolism in plants (12). However, the downstream components in the glucose-signaling pathway are mostly unknown. Although interactions between sugar and light or hormonal signaling pathways have been suggested (13–23), the mechanisms underlying the crosstalk between glucose and other signaling pathways remain obscure.
We report here the phenotypic, molecular, and genetic analyses of a recessive Arabidopsis mutant (glucose-insensitive, gin1) that is defective in many glucose-specific responses, including cotyledon greening and expansion, shoot development, floral transition, and gene expression. Genetic analysis indicates that gin1 is epistatic to HXK in the glucose-signaling pathway. Interestingly, we discovered that gin1 insensitivity to glucose repression of cotyledon and shoot development can be phenocopied by ethylene precursor treatment of wild-type plants or by constitutive ethylene mutants, whereas the ethylene insensitive mutant exhibits glucose hypersensitivity. Further characterizations suggest that GIN1 may act downstream of the glucose sensor and ethylene receptor to mediate the opposing roles that glucose and ethylene play in controlling developmental processes throughout the plant life cycle.
MATERIALS AND METHODS
Isolation of the gin1 Mutant.
Two A. thaliana ecotypes Wassilewskija (Ws) and Landsberg erecta (Ler) were the strains used for mutant isolation. T4 pools of T-DNA tagged lines or M2 populations from ethyl methanesulfonate (EMS)-mutagenized seeds were screened. Seeds were surface-sterilized and germinated on MS medium [Murashige and Skoog (MS) basal salts with B5 vitamins and 0.7% Phytoagar (pH 5.8)] containing 7% glucose under continuous fluorescent light (60 μE m−2 s−1) at 24°C. The use of 7% instead of 6% glucose eliminated false positives in the high density screen (2000 seedlings/150-mm plate). Putative glucose insensitive mutants that developed true leaves were transferred to pots containing Metro-Mix 200 (Scotts-Sierra, Marysville, OH) and grown under continuous light (60 μE m−2 s−1) for seed collection.
Genetic Analysis.
The gin1-1 mutant was backcrossed to the wild type (Ws-0), and the F1 seedlings were allowed to self-pollinate. F1 and F2 seedlings were scored for glucose sensitivity and greenhouse phenotype. The crosses between gin1-1 and gin1-2 were performed for complementation test. For mapping of the GIN1 locus, homozygous gin1-1 plants in the Ws background were crossed to wild-type plants of Columbia (Col-0) and Ler background. From the segregating F2 generation, 850 homozygous gin1-1 mutants were selected for mapping with simple sequence length polymorphism markers (24).
RNA Analysis.
For analysis of genes involved in photosynthesis, total RNA isolation and RNA blot analysis were performed as described (12).
Glucose Uptake Assay.
Glucose uptake assays of seedlings and protoplasts were performed as described (25, 26). Seedlings were grown in liquid MS medium with 2% glucose for 3 days. For each sample, five seedlings were incubated in MS medium and 2 μCi [14C]glucose (1 Ci = 37 GBq) in the presence of 1 mM unlabeled glucose for the indicated times. Uptake was terminated by washing in ice-cold buffer (5 mM Mes, pH 6.0) twice. The accumulated radioactivity was determined by liquid scintillation counting. Protoplasts were isolated as described (27). Approximately 2 × 106 protoplasts were added to the absorption solution (0.4 M mannitol/10 mM CaCl2/5 mM Mes, pH 6.0/2 μCi [14C]glucose/1 mM unlabeled glucose) and samples were taken at different time points. Protoplasts were washed in the absorption solution without glucose twice and glucose uptake was determined as above. Rates of glucose uptake were expressed as nmol per 104 protoplasts at various time points.
Immunoblot Analysis.
Arabidopsis plants were grown in liquid culture (half-strength MS basal salts, B5 vitamins, and 1% sucrose) for 2 weeks. Rosette tissues (100 mg) were ground in 500 μl 2× SDS/PAGE loading gel buffer (0.125 M Tris, pH 6.8/4% SDS/20% glycerol/0.78% DTT) and centrifuged. Equal amount of protein from each sample were separated on 10% SDS/PAGE and electrophoretically transferred to polvinylidene difluoride membrane. Immunodetection of HXK was performed as per the manufacturer’s instructions (New England BioLabs, Phototope-Star Western Blot Detection Kit) as described (12).
RESULTS
Isolation and Genetic Analysis of the gin1 Mutant.
Analysis of the glucose signal transduction pathway in higher plants has been facilitated by the discovery that light-grown Arabidopsis seedlings exhibit a dramatic developmental arrest specifically induced by elevated glucose levels (9, 12). In wild-type Arabidopsis plants, the greening and expansion of cotyledons and the initiation of true leaf development are normal when plants are grown on standard culture medium with MS salts and 2% glucose. However, these processes are completely suppressed when the glucose level is raised to 6% (12) (Fig. 1A). It has been shown that the developmental arrest induced by glucose can be exaggerated or eliminated by increasing or decreasing the levels of the specific glucose sensor (AtHXK) in transgenic Arabidopsis plants (12). More significantly, this glucose-dependent developmental arrest is uncoupled from glucose metabolism through the glycolytic pathway and tricarboxylic acid (TCA) cycle, as increasing the total hexose phosphorylation activity by overexpressing the heterologous yeast HXK2 in Arabidopsis did not confer plants more sensitive to the glucose-induced developmental arrest (12). Taking advantage of this striking and reproducible glucose response in early seedling development, we screened for Arabidopsis mutants displaying gin phenotypes. The putative gin mutants are likely to affect glucose signaling but not glucose metabolism through the glycolytic pathway and TCA cycle.
Figure 1.
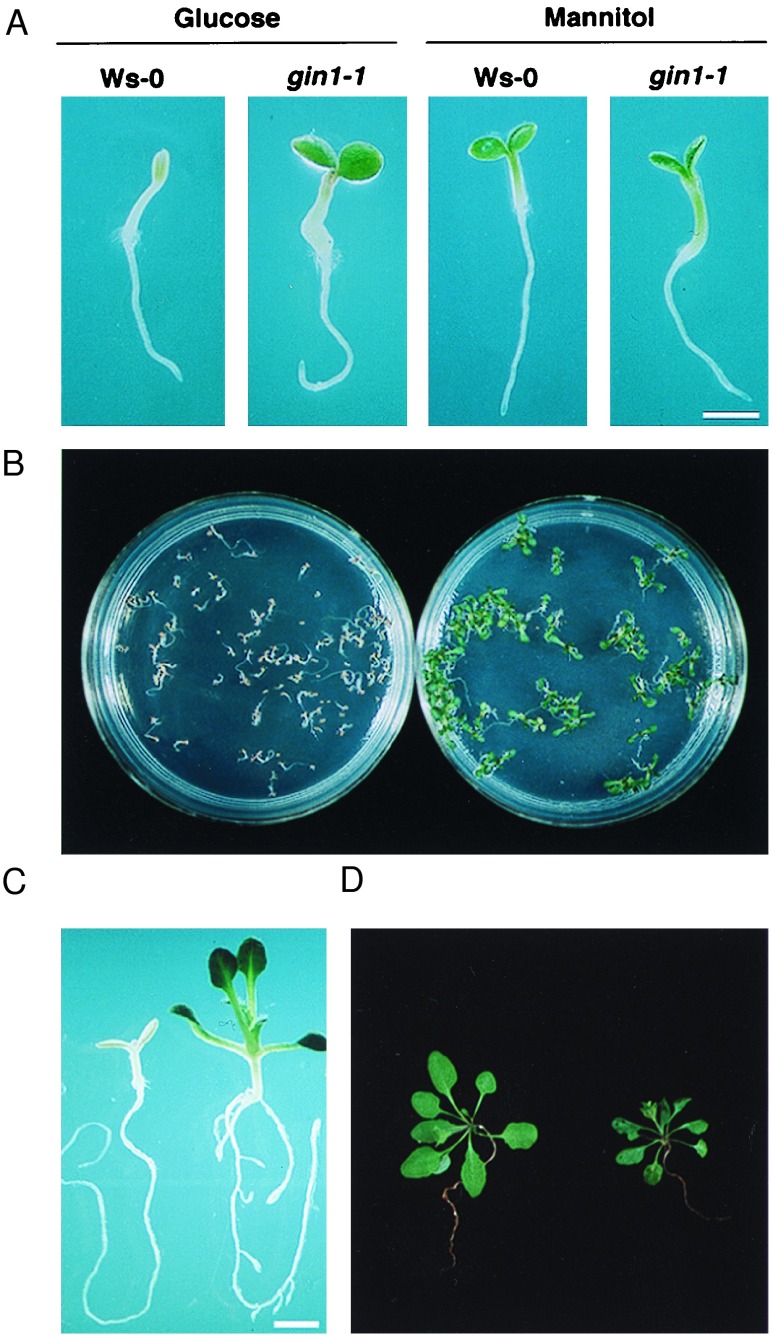
Phenotypes of gin1-1 plants. (A) Cotyledon development. gin1-1 and wild-type (Ws-0) seeds were germinated and grown on MS medium containing 6% glucose or mannitol for 4 days in the light (60 μE m−2 s−1). (Bar = 1.3 mm.) (B) The gin1-1 mutant population is uniformly insensitive to glucose. Approximately 150 seeds of wild type (Left) and gin1-1 (Right) were germinated and grown on MS medium containing 6% glucose for 4 days in the light. (C) True leaf development. Wild-type (Left) and gin1-1 (Right) seedlings were grown as in B for 10 days. (Bar = 1.9 mm.) (D) Greenhouse phenotype. Both wild-type (left) and gin1-1 (right) plants were grown in Metro-Mix 200 under continuous light (60 μE m−2 s−1) for 3 weeks.
A total of 80,000 seeds derived from the 6,500 independent T-DNA tagged lines generated by Kenneth Feldmann, Univ. of Arizona (ecotype Ws) and 30,000 EMS mutagenized M2 seeds (derived from 3,500 M1) of Ler were screened. Two gin1 alleles were found. gin1-1 was obtained from T-DNA pools and gin1-2 was from EMS pools. Genetic analysis indicated that the mutant phenotype was caused by a single recessive mutation (Table 1). Because both alleles showed similar phenotypes on glucose plates and in greenhouse, the studies in this paper were performed with the gin1-1 allele. gin1-1 developed green and expanded cotyledons in the presence of MS salts and 6% glucose (Fig. 1 A and B). However, the gin1-1 mutant exhibited morphology similar to the wild-type in the presence of 6% mannitol (Fig. 1A), suggesting that the gin1-1 mutation specifically affects responses to glucose but not to osmotic stress. The gin1-1 mutant but not the wild-type seedlings went on to develop true leaves and grow normally in the presence of 6% glucose (Fig. 1C). In the greenhouse, gin1-1 was shorter in stature, with smaller and darker-green rosettes, compared with wild type (Fig. 1D). These aberrant features of gin1-1 in the absence of exogenous glucose indicate that the gene product defined by GIN1 may play an essential role in the normal growth and development of Arabidopsis.
Table 1.
Genetic analysis of the gin1 mutant
| Cross | Type | Total | Mutant* | Wild Type | χ2† |
|---|---|---|---|---|---|
| GIN1/GIN1 × | F1 | 51 | 0 | 51 | |
| gin 1-1/gin 1-1 | F2 | 352 | 84 | 268 | 0.24‡ |
| GIN1/GIN1 × | F1 | 55 | 0 | 55 | |
| gin 1-2/gin 1-2 | F2 | 689 | 165 | 524 | 0.41‡ |
| gin1-1/gin1-1 × | F1 | 55 | 55 | 0 | |
| gin 1-2/gin 1-2 |
Mutant phenotypes include the glucose insensitivity on MS medium containing 6% glucose and greenhouse phenotypes.
The χ2 is given for the ratio of 3:1 (wild type/mutant).
Not significant at P = 0.05.
To map the GIN1 locus, gin1-1 in the Ws background was crossed to wild-type Col and Ler plants. From the F2 population, 850 gin1 homozygous plants were selected and genomic DNA was extracted from each plant. Initial mapping with simple sequence length polymorphism markers from each of the five chromosomes of Arabidopsis indicated that the gin1 mutation was tightly linked to nga280 and nga128 on chromosome 1 (only 9 recombinants were detected among the 850 plants, which is equivalent of 1700 chromosomes). Together with the analysis of the flanking markers using these recombinants (data not shown), GIN1 is located ≈0.6 cM on the centromeric side of nga280/nga128. GIN1 appears to define a new locus because other sucrose mutants (sun, rsr, lba) are mapped at distinct chromosome locations (20–22).
gin1 Is Insensitive to the Glucose Repression of Genes Involved in Photosynthesis.
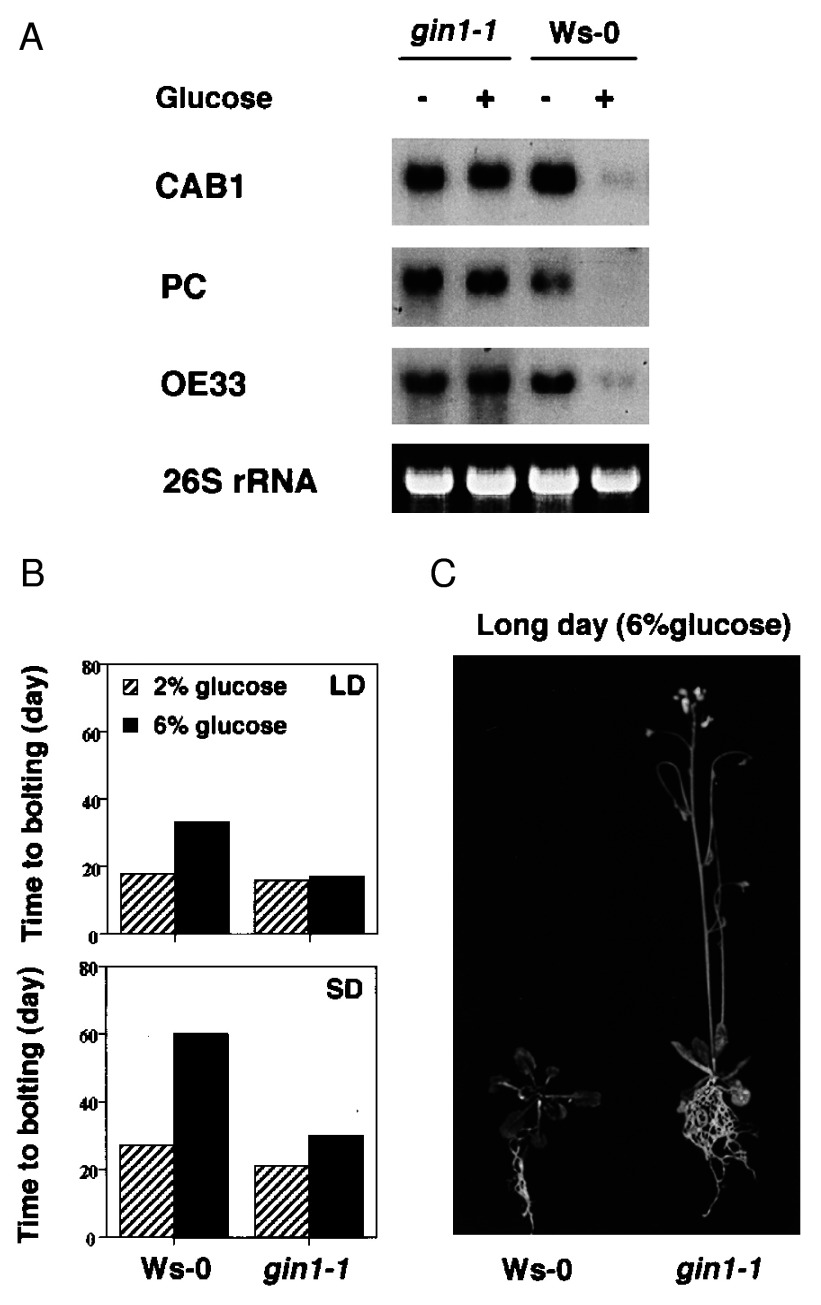
The expression of many genes involved in photosynthesis has been shown to be repressed by sugars in diverse plant species (4, 6–9, 13, 28). During early postembryonic growth of Arabidopsis, the transient activation of several genes important for photosynthesis is light independent but sugar repressible (19, 29). To avoid the morphological complications induced by light, we examined the specific effect of glucose on the expression of the chlorophyll a/b-binding protein (CAB1), plastocyanin (PC), and the 33-kDa oxygen-evolving polypeptide (OE33) genes (19, 29, 30) in dark-grown wild-type and gin1-1 plants. There was no difference in gene expression between the wild-type and gin1-1 plants in the absence of exogenous glucose. However, the presence of 4% glucose significantly reduced the expression of these three genes in wild type, but not in gin1-1 (Fig. 2A). This result suggests that GIN1 may play a regulatory role in glucose repression of multiple genes involved in photosynthesis.
Figure 2.
Reduced glucose sensitivity in gin1-1. (A) Glucose repression of photosynthetic genes. Total RNA was isolated from gin1-1 and wild-type (Ws-0) seedlings grown on MS medium containing 0 (−) or 4% glucose (+) in the dark for 3 days. RNA blot analysis was performed by using 5 μg of RNA per lane. Ribosomal RNA stained with ethidium bromide was used as a loading control. (B) Glucose effect on flowering. Lighting was continuous (60 μE m−2 s−1) for LD, or day/night cycles (8 hr/16 hr) for SD treatment. Bolting time was determined by the appearance of an inflorescence stem. Data were obtained from 10 replicates. Standard deviation was zero as all plants bolted within the same day under each condition. (C) Flowering is not delayed by glucose in gin1-1. gin1-1 and wild-type (Ws-0) plants were grown on MS medium with 6% glucose for 4 weeks under continuous light.
Flowering Time in gin1 Is Not Delayed by Glucose.
Sugars are known to affect flowering time in plants, although the mechanism is poorly understood (31, 32). We tested the effect of exogenous glucose on the time required for bolting of the inflorescence in gin1-1 and wild type. Seeds were first germinated in the presence of 2% glucose for 3 days before transfer to plantcons with 2% or 6% glucose-MS medium. Under constant white fluorescence light (LD), wild-type plants grown on 6% glucose medium bolted 16 days later than plants grown on 2% glucose medium. Under short day (SD) condition (8 hr light/16 hr dark), plants grown on high glucose bolted 30 days later than the plants grown on low glucose (Fig. 2B). The delay of flowering by high levels of glucose was accompanied by an overt increase of rosette leaf number from 8 (2%) to 20 (6%) under SD condition. In contrast, gin1-1 did not show a significant delay in bolting time when grown on 6% glucose plates under either conditions (LD or SD) (Fig. 2 B and C). The insensitivity of gin1-1 to the glucose-dependent delay of flowering was even detectable when both wild-type and mutant plants were grown on 2% glucose under SD conditions (Fig. 2B). Higher levels of glucose caused further delay of flowering in wild-type plants. Our study illustrates that the delay of flowering by glucose is mediated at least in part by GIN1.
gin1 Is Not Defective in Glucose Uptake.
To determine whether deficiency in glucose uptake can account for the gin1 phenotypes, we compared the uptake rates of glucose by using seedlings or protoplasts of both mutant and wild type. The rate of glucose uptake in gin1-1 was similar to that in wild type under both assay conditions (Fig. 3A). It is unlikely that the insensitivity of gin1-1 to glucose is caused by a deficiency in glucose transport.
Figure 3.
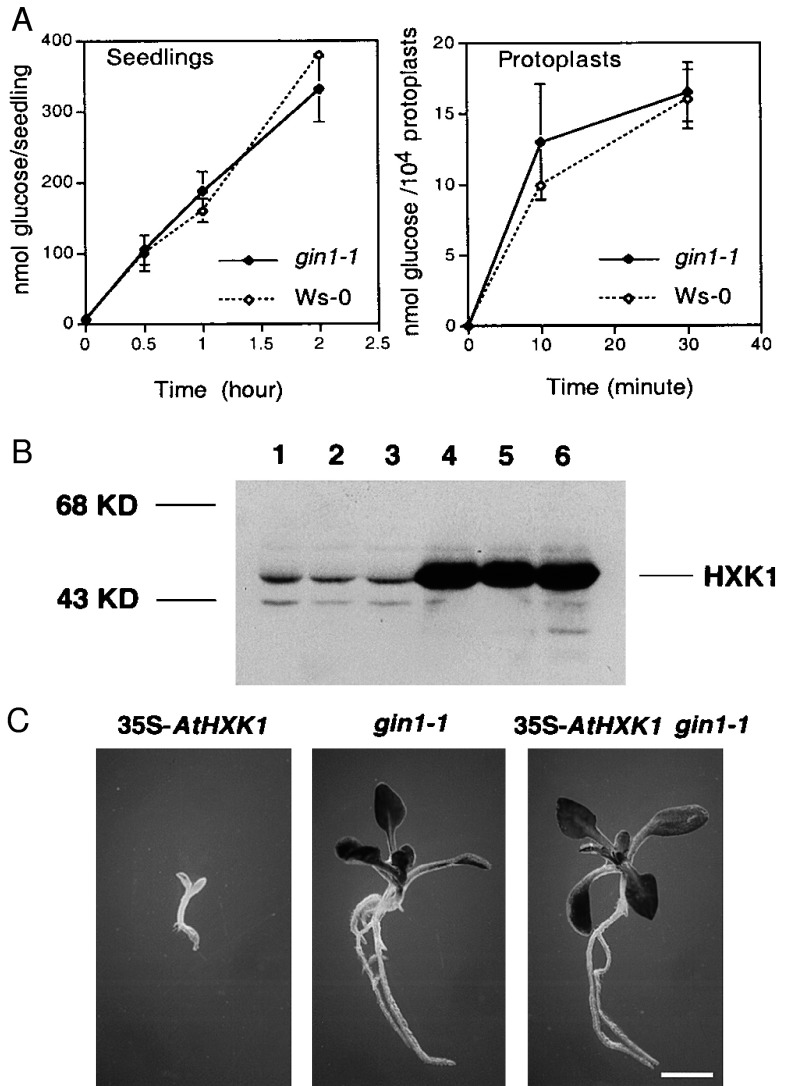
GIN1 as a positive regulator in the glucose signaling pathway mediated by HXK. (A) Glucose uptake. The rates of glucose uptake in the seedlings and protoplasts of gin1-1 (filled symbols) and wild-type (Ws-0) (opened symbols) plants was determined (10). Values are the means of three measurements (each with duplicated samples). Error bars indicate the standard deviation. (B) Immunoblot analysis of HXK. HXK levels were determined in wild-type Ws-0 (lane 1), gin1-1 (lane 2), wild-type Bensheim (lane 3), 35S-AtHXK1 transgenic plant (Bensheim ecotype) (lane 4), and two independent lines of 35S-AtHXK1 gin1-1 (lanes 5 and 6). Proteins were extracted from shoots of 14-day-old seedlings grown in liquid culture (11). (C) 35S-AtHXK1 gin1-1 can overcome the glucose induced developmental arrest. 35S-AtHXK1, gin1-1, and 35S-AtHXK1 gin1-1 seedlings were grown on MS medium with 6% glucose. (Bar = 1.9 mm.)
gin1 Is Epistatic to HXK in the Glucose Signaling Pathway.
We have previously shown that reducing HXK levels by overexpression of the antisense HXK genes in Arabidopsis diminished glucose sensitivity (12). Thus, it was important to determine whether gin1 is a HXK mutant. The protein level of HXK, determined by immunoblot analysis, was identical in wild type and gin1-1 (Fig. 3B). gin1-1 also had wild-type levels of HXK mRNA and total glucose phosphorylation activity (data not shown). Furthermore, gin1-1 was mapped to chromosome 1, whereas the two known Arabidopsis HXK genes are located on chromosomes 2 and 4 (12). The results indicate that gin1-1 is unlikely to be a mutant of HXK or regulator of HXK expression and activity.
To determine whether GIN1 plays a role in the glucose signaling pathway mediated by HXK, we generated a “double mutant” carrying gin1-1 and a transgene overexpressing an Arabidopsis HXK gene (35S-AtHXK1). The 35S-AtHXK1 transgenic plant have a high level of HXK and is hypersensitive to glucose, as described (12) (Fig. 3 B and C). Although the double mutant 35S-AtHXK gin1-1 plants had a high level of HXK protein (Fig. 3B), it showed the same glucose insensitive phenotype as gin1-1 grown on MS medium with 6% glucose (Fig. 3C). Consistently, 35S-AtHXK1 gin1-1 plants were similar to gin1-1 phenotypically, with small and dark-green rosettes when grown in the greenhouse (data not shown). These results suggest that GIN1 likely acts downstream of HXK in the HXK-mediated glucose signal transduction pathway.
Ethylene Plays a Role in the Glucose Insensitivity of gin1.
The phenotypes of gin1-1 provided an important clue to GIN1 function. For example, seeds of gin1-1 germinate faster (data not shown), and gin1-1 plants have smaller, darker-green rosettes (Fig. 1C) which are phenotypes induced by ethylene in the wild type and are found in constitutive ethylene response mutants (33–43). We were interested in understanding whether this phenotypic convergence was indicative of a relationship between the glucose and the ethylene signaling pathways. Wild-type seeds (Ws-0 and Col-0) were germinated on MS medium with 6% glucose. To enhance ethylene production from the seedlings, the immediate ethylene precursor, 1-aminocyclopropane-1-carboxylic acid (ACC), which is readily converted to ethylene in plants (38), was added into the glucose plates. As shown in Fig. 4A, wild-type seedlings treated with ACC could overcome the developmental arrest normally induced by glucose. This result reveals an unconventional finding that ethylene may antagonize glucose signals to promote cotyledon and shoot development in wild-type seedlings.
Figure 4.
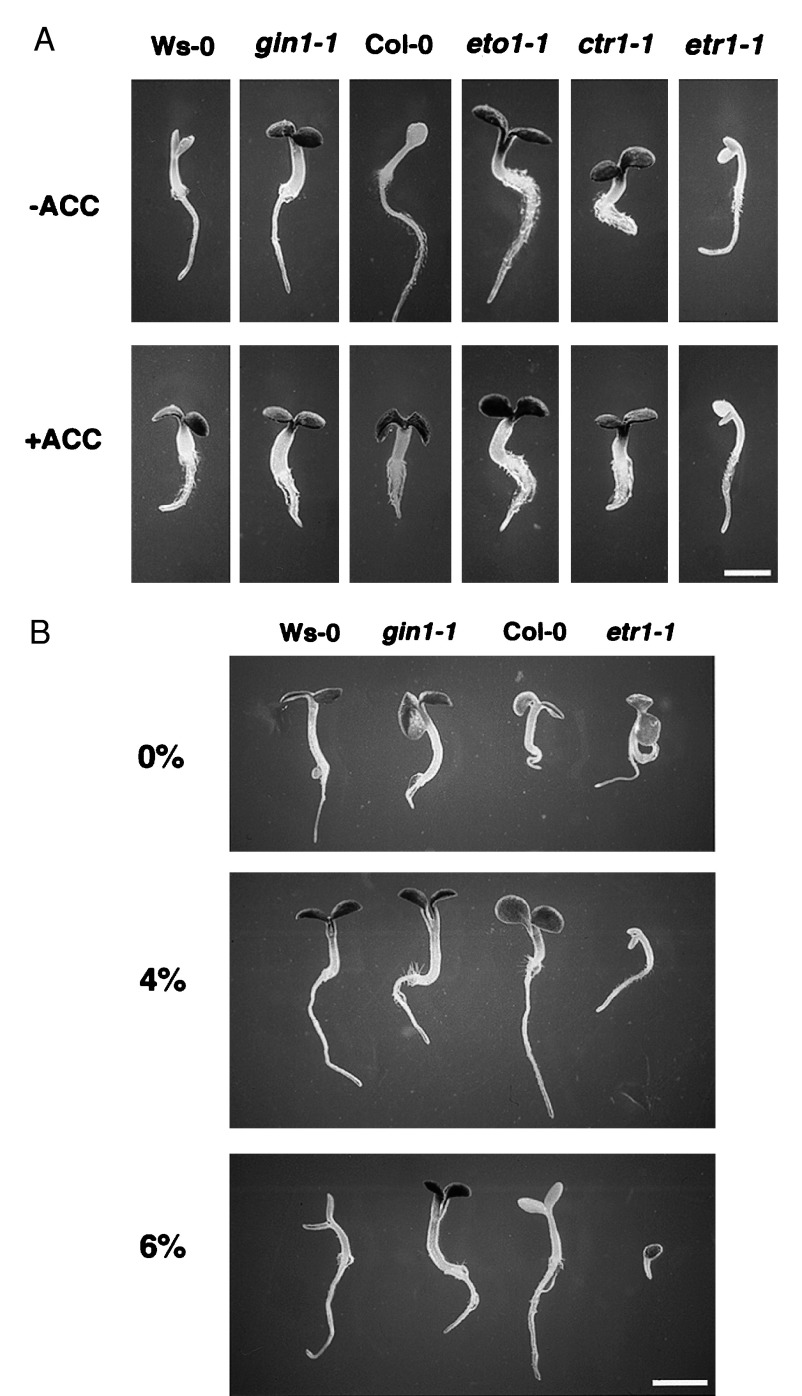
Crosstalk between ethylene and glucose signaling. (A) ACC treatment can phenocopy gin1 phenotype. Glucose-induced developmental arrest was examined in wild-type seedlings, gin1-1, and ethylene mutants on MS medium containing 6% glucose in the absence (Upper) or presence (Lower) of 50 μM of ACC. Wild-type (Ws-0) and gin1-1 seedlings were 4 days old and all others were 5 days old. (Bar = 1.3 mm.) (B) etr1-1 is hypersensitive to glucose. Wild-type, gin1-1, and etr1-1 seeds were germinated and grown on MS medium containing 0%, 4% or 6% glucose for 4 days in the light (60 μE m−2 s−1). (Bar = 1.3 mm.)
Ethylene Mutants Show Altered Glucose Sensitivity.
To further test the involvement of ethylene in glucose signaling, we analyzed the glucose sensitivity of three classes of ethylene mutants: the ethylene overproduction mutant eto1-1, the constitutive ethylene triple response mutant ctr1-1, and the ethylene-insensitive mutant etr1-1 (35–43). Both eto1-1 and ctr1-1 overcame the glucose-induced developmental arrest without ACC treatment, whereas etr1-1 remained glucose sensitive even in the presence of ACC (Fig. 4A). These data provide the most compelling evidence that glucose sensitivity is tightly linked to the ethylene signaling pathway.
The etr1-1 Mutant Displays Glucose Hypersensitivity.
Interestingly, etr1-1 appeared to be more sensitive to glucose than the wild type (Fig. 4A). We carried out a comparison between the etr1-1 and wild-type plants at different concentrations of glucose. No overt difference in cotyledon development was found in the absence of exogenous glucose (Fig. 4B). However, in the presence of 3–4% glucose, etr1-1 already displayed the developmental arrest that could only be found in the wild-type seedlings in the presence of 6% glucose (Fig. 4B). At an earlier stage, the germination of etr1-1 seeds was severely retarded in the presence of 6% glucose (Fig. 4B). These results provide further supporting evidence that both ethylene and glucose play important, possibly opposite, roles in germination and the development of cotyledons and true leaves.
GIN1 Acts Downstream of ETR1.
To examine further the role of GIN1 in the ethylene signal transduction pathway, the etr1-1 gin1-1 double mutant was generated and analyzed. The dominant etr1-1 mutation in the ethylene receptor confers ethylene insensitivity (35, 37), and its presence in the double mutant was confirmed by DNA sequencing (data not shown). Unlike etr1-1, the etr1-1 gin1-1 seedlings could overcome the developmental arrest induced by 6% glucose (Fig. 5A), indicating that GIN1 or the target of GIN1 acts downstream of ETR1 in the ethylene signaling pathway. In the greenhouse, the double mutant also showed gin1-1, but not etr1-1, phenotypes. For instance, etr1-1 gin1-1 plants were dark-green with small rosettes (<50% of etr1-1), and flowered 2 weeks earlier than etr1-1 under constant light (Fig. 5B). These results provide further evidence that gin1-1 represents a rare constitutive ethylene signaling mutant and GIN1 may function to mediate the opposing roles of glucose and ethylene in controlling developmental processes throughout the plant life cycle.
Figure 5.
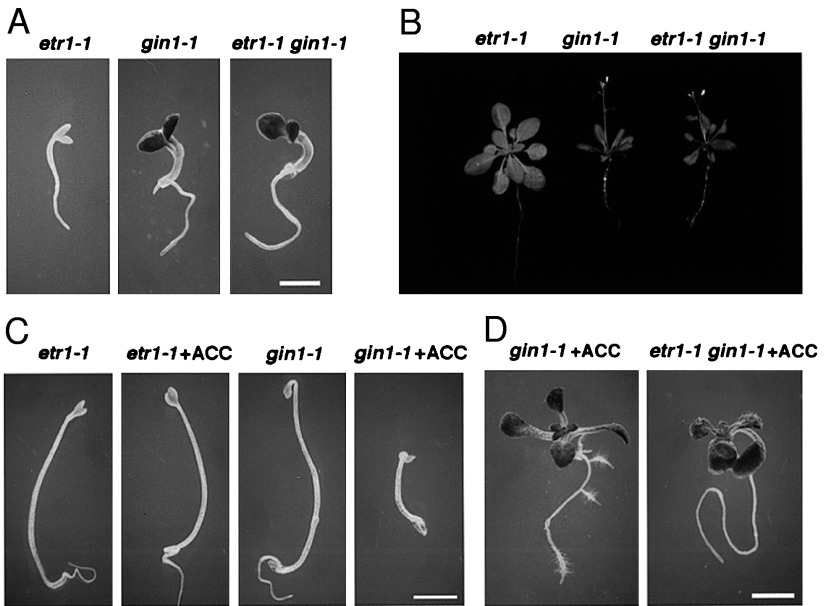
Phenotypes of the etr1-1 gin1-1 double mutant. (A) gin1-1 is epistatic to etr1-1. etr1-1, gin1-1, and etr1-1 gin1-1 seedlings were grown on MS medium containing 6% glucose for 5 days under constant light. (Bar = 1.3 mm.) (B) Greenhouse phenotype of etr1-1 gin1-1 Four-week-old etr1-1, gin1-1, and etr1-1 gin1-1 plants grown in the greenhouse are shown. (C) The GIN1 pathway is uncoupled from the ethylene triple response. etr1-1 and gin1-1 seedlings were grown on MS medium containing 1% sucrose in the absence or presence of 50 μM ACC for 3 days in the dark. (Bar = 1.6 mm.) (D) The GIN1 pathway is genetically separable from the ethylene root hair response. gin1-1 and etr1-1 gin1-1 seedlings from A were transferred to MS medium containing 1% sucrose and 50 μM ACC for an additional 2 days. (Bar = 1.9 mm.)
Surprisingly, the typical ethylene-induced triple response, including shortening and radial swelling of the hypocotyl, inhibition of root elongation, and exaggerated curvature of the apical hook in dark-grown seedlings, was not observed in gin1-1 grown in the absence of ACC in the dark (Fig. 5C). As only a portion of the ethylene regulatory circuits appeared to be perturbed in gin1-1, we expected that gin1-1 would show some ethylene phenotypes not found in the etr1-1 gin1-1 double mutant. When treated with ACC, gin1-1 showed additional ethylene responses, such as inhibition of root elongation and stimulation of root hair formation (44), whereas etr1-1 gin1-1 seedlings did not exhibit more ethylene phenotypes in the presence of ACC (Fig. 5D). Thus, GIN1 plays a role in a branch of ethylene signaling pathway that can be uncoupled from the ethylene signaling pathway controlling root hair initiation and the triple response exhibited in the other constitutive ethylene signaling mutant ctr1-1 (43). Mutations in the GIN1-specific pathway cannot be recovered from the previous ethylene mutant selection based on the triple response.
DISCUSSION
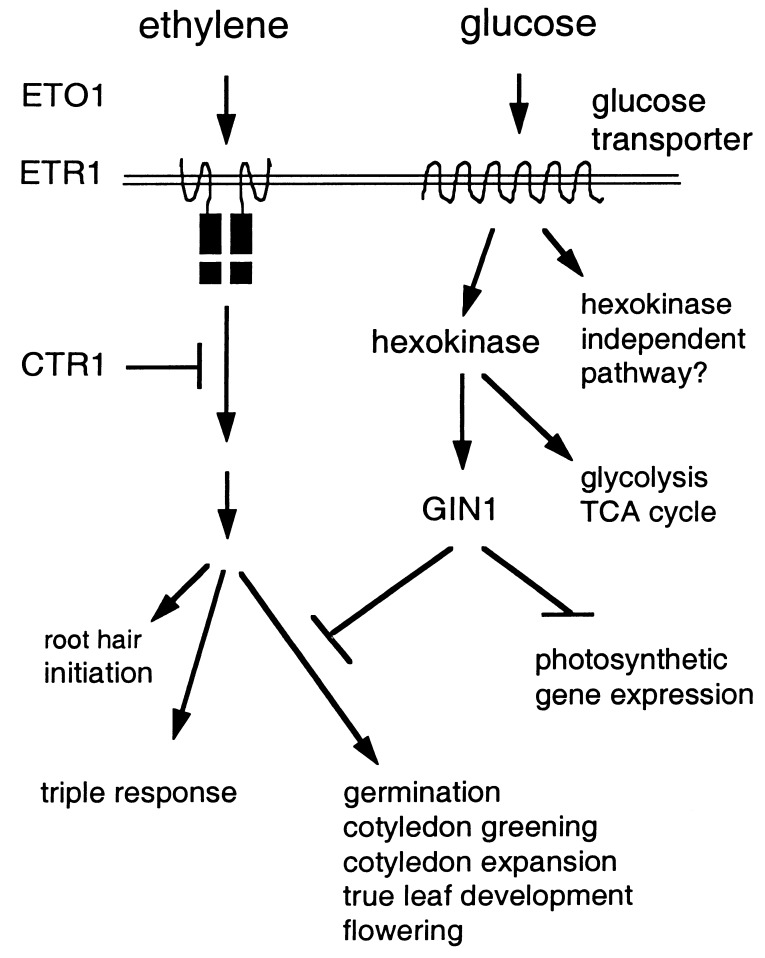
In addition to its roles as a major energy source and structural/storage component, glucose has recently been shown to act as a regulatory molecule in higher plants (4–9). Through genetic and phenotypic analyses of a glucose insensitive mutant (gin1) in Arabidopsis, we have shown that GIN1 defines a positive regulator that plays a pivotal role in the glucose repression of germination, cotyledon greening and expansion, true leaf development, floral transition, and gene expression (Fig. 6). The physiological importance of glucose regulation is manifested by the pleiotropic phenotypes of the gin1 mutant grown in the absence of exogenous glucose. By using distinct strategies and sucrose-dependent gene expression, other types of sugar mutants (e.g., sun, rsr, lba, hba) also have been isolated from Arabidopsis (19–23). The genetic, phenotypic, and molecular characterization of these distinct mutants will offer a new opportunity to elucidate the complex signaling networks controlled by different sugar signals in higher plants.
Figure 6.
Model for glucose and ethylene signaling.
HXK as the glucose sensor appears to be evolutionarily conserved in bacteria, yeast, mammals, and higher plants. However, in multicellular eukaryotes, little is known about the downstream components in the glucose signal transduction pathways. Based on the analysis of transgenic Arabidopsis plants carrying distinct HXK genes, it has been proposed that there may be HXK-dependent pathways and HXK-independent pathways in plants (9) (Fig. 6). The cross between HXK overexpressor lines and gin1-1 showed that the double mutant gives totally gin1 phenotype, both on high glucose plate and in soil. This result indicates that gin1 is the first glucose signaling mutant that defines a positive regulator acting downstream of the HXK-dependent signaling pathway (Fig. 6).
Most surprisingly, GIN1 seems to execute, in a glucose signal-dependent manner, some of its functions by antagonizing a branch of the ethylene signal transduction pathway that is uncoupled from the typical ethylene triple response. Thus, only a branch of the ethylene signaling pathway is altered in gin1-1. The GIN1 pathway is genetically separable from the pathway conferring other ethylene responses (Fig. 6). The role of ethylene in photosynthetic gene expression is unclear and will require further investigation (Fig. 6). Ethylene is best known as a plant growth regulator that controls fruit-ripening and senescence (33–43). However, recent studies have added numerous physiological roles of ethylene in cell elongation and expansion, root hair development, pathogen defense, wounding, and nodulation (44–47). The characterization of gin1 mutant has revealed an unexpected interplay between the glucose and ethylene signaling pathways in coordinated regulation of many developmental transitions in Arabidopsis. Besides environmental and hormonal signals, the perception and transmission of metabolic signals also contribute to the regulation of plant growth and development through intertwined signaling networks in higher plants.
Acknowledgments
We thank E. Schaller for generous supply of ethylene and advice. We also thank Y. Kovtun, Y. Lin, J. Nardone, J. Nishio, and G. Ruvkun for critical reading of the manuscript, Arabidopsis Biological Resource Center for mutant seeds, the National Science Foundation (Grants IBN-9221086 and IBN-9723610), and Hoechst A.G. for funding. T.L.J. is supported by a postdoctoral fellowship from the National Science Foundation.
ABBREVIATIONS
- HXK
hexokinase
- gin
glucose insensitive
- MS medium
Murashige and Skoog medium
- EMS
ethyl methanesulfonate
- ACC
1-aminocyclopropane-1-carboxylic acid
- eto
ethylene overproduction
- ctr
constitutive triple response
- etr
ethylene insensitive
References
- 1.Saier M H, Jr, Chauvaux S, Deutscher J, Reizer J, Ye J-J. Trends Biochem Sci. 1995;20:267–271. doi: 10.1016/s0968-0004(00)89041-6. [DOI] [PubMed] [Google Scholar]
- 2.Ronne H. Trends Genet. 1995;11:12–17. doi: 10.1016/s0168-9525(00)88980-5. [DOI] [PubMed] [Google Scholar]
- 3.Newgard C B, McGarry J D. Annu Rev Biochem. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- 4.Sheen J. Photosynth Res. 1994;39:427–438. doi: 10.1007/BF00014596. [DOI] [PubMed] [Google Scholar]
- 5.Thomas B, R, Rodriguez R L. Plant Physiol. 1994;106:1235–1239. doi: 10.1104/pp.106.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stitt M, Sonnewald U. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:341–368. [Google Scholar]
- 7.Koch K E. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- 8.Smeekens S, Rook F. Plant Physiol. 1997;115:7–13. doi: 10.1104/pp.115.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang J C, Sheen J. Trends Plant Sci. 1997;2:208–213. [Google Scholar]
- 10.Graham I A, Denby K J, Leaver C J. Plant Cell. 1994;4:761–772. doi: 10.1105/tpc.6.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang J C, Sheen J. Plant Cell. 1994;6:1665–1679. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang J C, Leon P, Zhou L, Sheen J. Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheen J. Plant Cell. 1990;2:1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason H S, DeWald D B, Creelman R A, Mullet J E. Plant Physiol. 1992;98:859–867. doi: 10.1104/pp.98.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeWald D B, Sadka A, Mullet J E. Plant Physiol. 1994;104:439–444. doi: 10.1104/pp.104.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai C, Miller A, Spalding M, Rodermel S. Plant Physiol. 1997;115:907–914. doi: 10.1104/pp.115.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faure J D, Jullien M, Caboche M. Plant J. 1994;5:481–491. doi: 10.1046/j.1365-313x.1994.5040481.x. [DOI] [PubMed] [Google Scholar]
- 18.Perata P, Matsukura C, Vernieri P, Yamaguchi J. Plant Cell. 1997;9:2197–2208. doi: 10.1105/tpc.9.12.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dijkwel P P, Huijser C, Weisbeek P J, Chua N-H, Smeekens S. Plant Cell. 1997;9:583–595. doi: 10.1105/tpc.9.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Oosten J J, Gerbaud A, Huijser C, Dijkwel P P, Chua N H. Plant J. 1997;12:1011–1020. doi: 10.1046/j.1365-313x.1997.12051011.x. [DOI] [PubMed] [Google Scholar]
- 21.Martin T, Hellmann H, Schmidt R, Willmitzer L, Frommer W. Plant J. 1997;11:53–62. doi: 10.1046/j.1365-313x.1997.11010053.x. [DOI] [PubMed] [Google Scholar]
- 22.Mita S, Murano N, Akaike M, Nakamura K. Plant J. 1997;11:841–851. doi: 10.1046/j.1365-313x.1997.11040841.x. [DOI] [PubMed] [Google Scholar]
- 23.Mita S, Hirano H, Nakamura K. Plant Physiol. 1997;114:572–582. doi: 10.1104/pp.114.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell C J, Ecker J R. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- 25.Thorne J H. Plant Physiol. 1982;70:953–958. doi: 10.1104/pp.70.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giaquinta R T, Lin W, Sadler N L, Franceschi V R. Plant Physiol. 1983;72:362–367. doi: 10.1104/pp.72.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheen J. Plant Cell. 1991;3:225–245. doi: 10.1105/tpc.3.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng Chi-Lien, Acedo G N, Cristinsin M, Conkling M A. Proc Natl Acad Sci USA. 1992;89:1961–1864. doi: 10.1073/pnas.89.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brusslan J A, Tobin E M. Proc Natl Acad Sci USA. 1992;89:7791–7795. doi: 10.1073/pnas.89.16.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson E J, Leech R M. Plant Physiol. 1995;107:63–71. doi: 10.1104/pp.107.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernier G, Havelange A, Houssa C, Petitjean A, Lejeune P. Plant Cell. 1993;5:1147–1155. doi: 10.1105/tpc.5.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eimert K, Wang S-M, Lue W-L, Chen J. Plant Cell. 1995;7:1703–1712. doi: 10.1105/tpc.7.10.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ketring D L. In: Ethylene and Seed Germination. Warding P F, editor. London: Academic; 1977. pp. 157–178. [Google Scholar]
- 34.Yang S F, Hoffman N E. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- 35.Bleecker A B, Estelle M, Somerville C, Kende H. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- 36.Guzman P, Ecker J. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang C, Kwok S, Bleecker A, Meyerowitz E. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- 38.Kende H. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. [Google Scholar]
- 39.Grbic V, Bleecker A B. Plant J. 1995;8:595–602. [Google Scholar]
- 40.Ecker J. Science. 1995;268:667–675. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- 41.Bleecker A B, Schaller G E. Plant Physiol. 1996;111:653–660. doi: 10.1104/pp.111.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang C. Trends Biochem Sci. 1996;21:129–133. [PubMed] [Google Scholar]
- 43.Kieber J. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:277–297. doi: 10.1146/annurev.arplant.48.1.277. [DOI] [PubMed] [Google Scholar]
- 44.Tanimoto M, Roberts K, Dolan L. Plant J. 1995;8:943–948. doi: 10.1046/j.1365-313x.1995.8060943.x. [DOI] [PubMed] [Google Scholar]
- 45.Knoester M, van Loon L C, van den Heuvel J, Hennig J, Bol J F, Linthorst H J M. Proc Natl Acad Sci USA. 1998;95:1933–1937. doi: 10.1073/pnas.95.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Donnell P J, Calvert C, Atzorn R, Wasternack C, Leyser H M O, Bowles D J. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- 47.Penmetsa R V, Cook D R. Science. 1997;275:527–523. doi: 10.1126/science.275.5299.527. [DOI] [PubMed] [Google Scholar]