Abstract
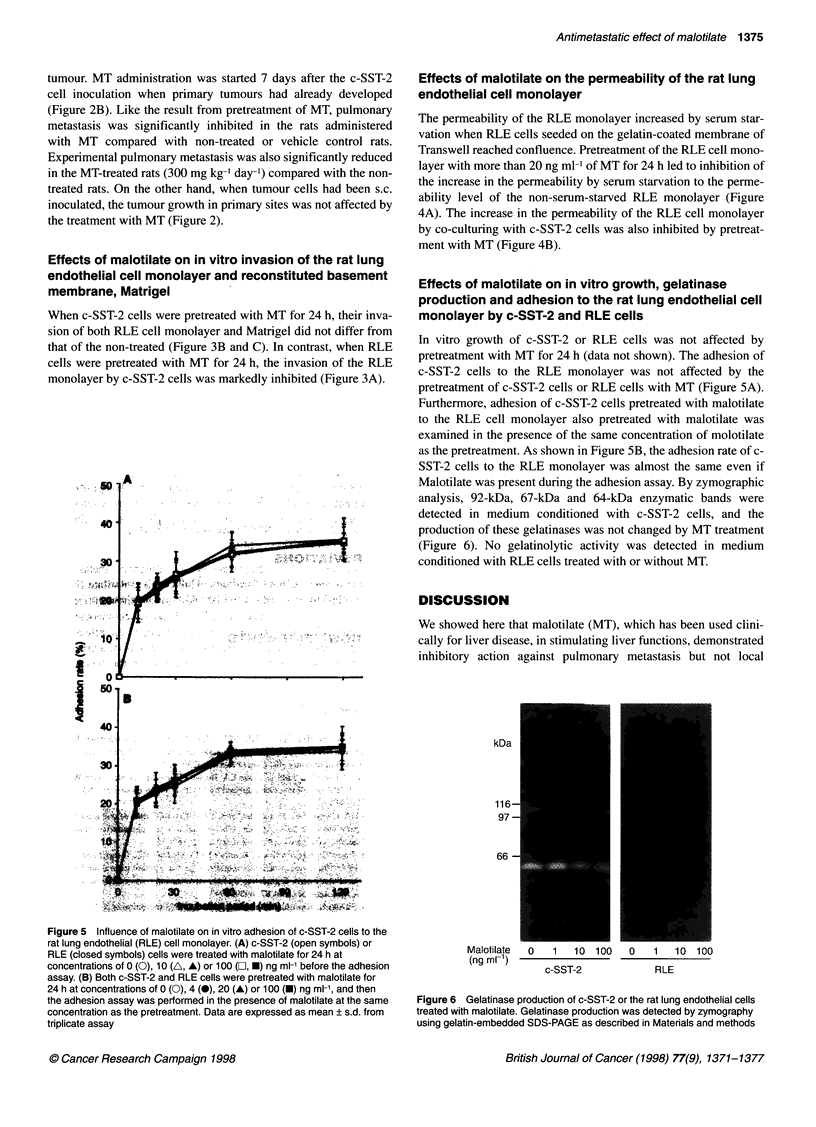
Malotilate (diisopropyl,1,3-dithiol-2-ylidenemalonate, MT) is clinically used as a hepatoprotective agent. Because we noticed that MT induced the differentiation of cultured vascular endothelial cells, we have examined its effects on lung metastasis of the highly metastatic rat mammary carcinoma c-SST-2. MT was orally administered to syngeneic SHR rats from 7 days before or after s.c. inoculation of c-SST-2 cells to the end of the experiments. In the MT-treated rats, pulmonary metastasis was markedly suppressed compared with the non-treated rats. In the rats treated with MT for 19 days after i.v. inoculation of c-SST-2 cells, lung metastasis was also significantly suppressed. An in vitro invasion assay using a rat lung endothelial (RLE) cell monolayer revealed that pretreatment of the RLE cells with MT, but not c-SST-2 cells, significantly reduced the invasion of the RLE monolayer by c-SST-2 cells. An in vitro vascular permeability assay demonstrated that MT prevented the increase in permeability of the RLE monolayer by serum starvation. On the other hand, in vivo and in vitro growth, gelatinase production and adhesion to the RLE cell monolayer of c-SST-2 cells were not affected by MT treatment. These findings suggest that MT suppressed tumour metastasis by intensifying the cell-to-cell contact of endothelial cells, thus preventing tumour cells from invading vascular endothelium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Iwamoto Y., Kleinman H. K., Martin G. R., Aaronson S. A., Kozlowski J. M., McEwan R. N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987 Jun 15;47(12):3239–3245. [PubMed] [Google Scholar]
- Albini A., Melchiori A., Santi L., Liotta L. A., Brown P. D., Stetler-Stevenson W. G. Tumor cell invasion inhibited by TIMP-2. J Natl Cancer Inst. 1991 Jun 5;83(11):775–779. doi: 10.1093/jnci/83.11.775. [DOI] [PubMed] [Google Scholar]
- Alvarez O. A., Carmichael D. F., DeClerck Y. A. Inhibition of collagenolytic activity and metastasis of tumor cells by a recombinant human tissue inhibitor of metalloproteinases. J Natl Cancer Inst. 1990 Apr 4;82(7):589–595. doi: 10.1093/jnci/82.7.589. [DOI] [PubMed] [Google Scholar]
- Cajot J. F., Bamat J., Bergonzelli G. E., Kruithof E. K., Medcalf R. L., Testuz J., Sordat B. Plasminogen-activator inhibitor type 1 is a potent natural inhibitor of extracellular matrix degradation by fibrosarcoma and colon carcinoma cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6939–6943. doi: 10.1073/pnas.87.18.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S., Suzuki H., Kinoshita M., Hirohashi S. Inhibition of cell attachment, invasion and metastasis of human carcinoma cells by anti-integrin beta 1 subunit antibody. Jpn J Cancer Res. 1992 Dec;83(12):1317–1326. doi: 10.1111/j.1349-7006.1992.tb02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada J., Takeichi N., Kobayashi H. Metastatic capacity and intercellular communication between normal cells and metastatic cell clones derived from a rat mammary carcinoma. Cancer Res. 1988 Sep 15;48(18):5129–5132. [PubMed] [Google Scholar]
- Humphries M. J., Olden K., Yamada K. M. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986 Jul 25;233(4762):467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Katoh M., Sugimoto T., Kasai T. Effect of malotilate (diisopropyl 1,3-dithiol-2-ylidenemalonate) on the protein synthesis in rat liver. Jpn J Pharmacol. 1982 Apr;32(2):369–375. doi: 10.1254/jjp.32.369. [DOI] [PubMed] [Google Scholar]
- Ingber D., Fujita T., Kishimoto S., Sudo K., Kanamaru T., Brem H., Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990 Dec 6;348(6301):555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y., Robey F. A., Graf J., Sasaki M., Kleinman H. K., Yamada Y., Martin G. R. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987 Nov 20;238(4830):1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- Izumi Y., Taniuchi Y., Tsuji T., Smith C. W., Nakamori S., Fidler I. J., Irimura T. Characterization of human colon carcinoma variant cells selected for sialyl Lex carbohydrate antigen: liver colonization and adhesion to vascular endothelial cells. Exp Cell Res. 1995 Jan;216(1):215–221. doi: 10.1006/excr.1995.1027. [DOI] [PubMed] [Google Scholar]
- Katoh M., Sugimoto T. [Effect of malotilate (diisopropyl 1, 3-dithiol-2-ylidenemalonate) on chronic liver injury caused by carbon tetrachloride]. Nihon Yakurigaku Zasshi. 1982 Jul;80(1):83–91. [PubMed] [Google Scholar]
- Kawaguchi S., Kikuchi K., Ishii S., Takada Y., Kobayashi S., Uede T. VLA-4 molecules on tumor cells initiate an adhesive interaction with VCAM-1 molecules on endothelial cell surface. Jpn J Cancer Res. 1992 Dec;83(12):1304–1316. doi: 10.1111/j.1349-7006.1992.tb02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokha R. Suppression of the tumorigenic and metastatic abilities of murine B16-F10 melanoma cells in vivo by the overexpression of the tissue inhibitor of the metalloproteinases-1. J Natl Cancer Inst. 1994 Feb 16;86(4):299–304. doi: 10.1093/jnci/86.4.299. [DOI] [PubMed] [Google Scholar]
- Khokha R., Zimmer M. J., Graham C. H., Lala P. K., Waterhouse P. Suppression of invasion by inducible expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in B16-F10 melanoma cells. J Natl Cancer Inst. 1992 Jul 1;84(13):1017–1022. doi: 10.1093/jnci/84.13.1017. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Gotoh J., Kanayama N., Hirashima Y., Terao T., Sugino D. Inhibition of tumor cell invasion through matrigel by a peptide derived from the domain II region in urinary trypsin inhibition. Cancer Res. 1995 May 1;55(9):1847–1852. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Gene products which play a role in cancer invasion and metastasis. Breast Cancer Res Treat. 1988 May;11(2):113–124. doi: 10.1007/BF01805835. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Mueller B. M., Yu Y. B., Laug W. E. Overexpression of plasminogen activator inhibitor 2 in human melanoma cells inhibits spontaneous metastasis in scid/scid mice. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):205–209. doi: 10.1073/pnas.92.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., Lotan D., Baig M. M., Carralero R. M., Wood W. R., Hendrix M. J., Lotan R. Inhibition by retinoic acid of type IV collagenolysis and invasion through reconstituted basement membrane by metastatic rat mammary adenocarcinoma cells. Cancer Res. 1989 Apr 1;49(7):1698–1706. [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta. 1982 Dec 21;695(2):113–176. doi: 10.1016/0304-419x(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis: tumor cell and host organ properties important in metastasis to specific secondary sites. Biochim Biophys Acta. 1988 Nov 15;948(2):175–224. doi: 10.1016/0304-419x(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Metastatic tumor cell interactions with endothelium, basement membrane and tissue. Curr Opin Cell Biol. 1989 Oct;1(5):1009–1019. doi: 10.1016/0955-0674(89)90073-2. [DOI] [PubMed] [Google Scholar]
- Nokata M., Katoh M., Sugimoto T. Protective effect of malotilate (diisopropyl 1,3-dithiol-2-ylidenemalonate) on carbon tetrachloride-induced liver injury in mice and rats. J Toxicol Sci. 1985 Nov;10(4):279–288. doi: 10.2131/jts.10.279. [DOI] [PubMed] [Google Scholar]
- O'Reilly M. S., Holmgren L., Shing Y., Chen C., Rosenthal R. A., Moses M., Lane W. S., Cao Y., Sage E. H., Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994 Oct 21;79(2):315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Ohigashi H., Shinkai K., Mukai M., Ishikawa O., Imaoka S., Iwanaga T., Akedo H. In vitro invasion of endothelial cell monolayer by rat ascites hepatoma cells. Jpn J Cancer Res. 1989 Sep;80(9):818–821. doi: 10.1111/j.1349-7006.1989.tb01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli B. U., Augustin-Voss H. G., el-Sabban M. E., Johnson R. C., Hammer D. A. Organ-preference of metastasis. The role of endothelial cell adhesion molecules. Cancer Metastasis Rev. 1990 Nov;9(3):175–189. doi: 10.1007/BF00046359. [DOI] [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Ryle P. R., Dumont J. M. Malotilate: the new hope for a clinically effective agent for the treatment of liver disease. Alcohol Alcohol. 1987;22(2):121–141. [PubMed] [Google Scholar]
- Saiki I., Iida J., Murata J., Ogawa R., Nishi N., Sugimura K., Tokura S., Azuma I. Inhibition of the metastasis of murine malignant melanoma by synthetic polymeric peptides containing core sequences of cell-adhesive molecules. Cancer Res. 1989 Jul 15;49(14):3815–3822. [PubMed] [Google Scholar]
- Schultz R. M., Silberman S., Persky B., Bajkowski A. S., Carmichael D. F. Inhibition by human recombinant tissue inhibitor of metalloproteinases of human amnion invasion and lung colonization by murine B16-F10 melanoma cells. Cancer Res. 1988 Oct 1;48(19):5539–5545. [PubMed] [Google Scholar]
- Sunada H., Masuda M., Fujiwara K. Preservation of differentiated phenotypes in cultured aortic endothelial cells by malotilate and phosphoascorbic acid. Eur J Cell Biol. 1993 Feb;60(1):48–56. [PubMed] [Google Scholar]
- Tang D. G., Diglio C. A., Honn K. V. 12(S)-HETE-induced microvascular endothelial cell retraction results from PKC-dependent rearrangement of cytoskeletal elements and alpha V beta 3 integrins. Prostaglandins. 1993 Mar;45(3):249–267. doi: 10.1016/0090-6980(93)90051-8. [DOI] [PubMed] [Google Scholar]
- Tang D. G., Timar J., Grossi I. M., Renaud C., Kimler V. A., Diglio C. A., Taylor J. D., Honn K. V. The lipoxygenase metabolite, 12(S)-HETE, induces a protein kinase C-dependent cytoskeletal rearrangement and retraction of microvascular endothelial cells. Exp Cell Res. 1993 Aug;207(2):361–375. doi: 10.1006/excr.1993.1203. [DOI] [PubMed] [Google Scholar]
- Zijlstra F. J., Wilson J. H., Vermeer M. A., Ouwendijk R. J., Vincent J. E. Differential effects of malotilate on 5-, 12- and 15-lipoxygenase in human ascites cells. Eur J Pharmacol. 1989 Jan 17;159(3):291–295. doi: 10.1016/0014-2999(89)90160-x. [DOI] [PubMed] [Google Scholar]