Abstract
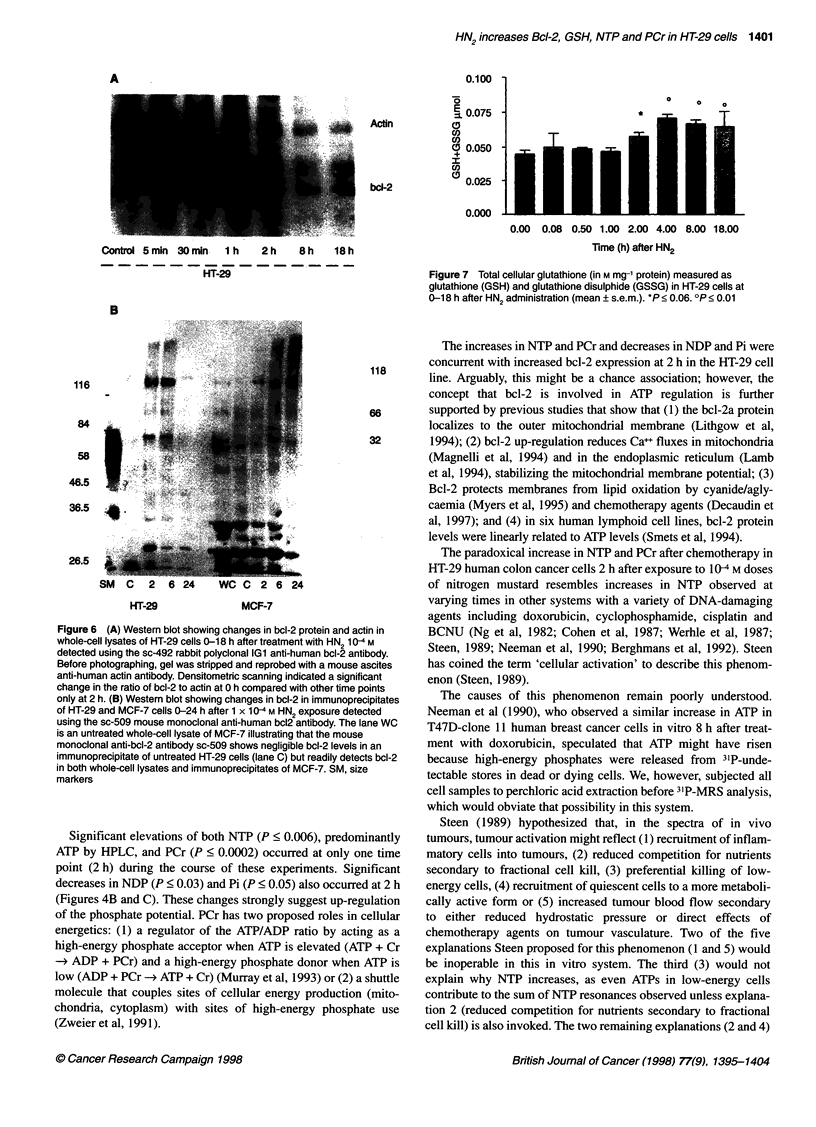
We hypothesized that unexplained increases in nucleoside triphosphates (NTP) observed by 31P magnetic resonance spectroscopy (MRS) after treatment of tumours by DNA-damaging agents were related to chemotherapy-induced up-regulation of the bcl-2 gene and DNA damage prevention and repair processes. To test this hypothesis, we treated HT-29 cells with 10(-4) M nitrogen mustard (HN2) and performed sequential perchloric acid extractions in replicate over 0-18 h. By reference to an internal standard (methylene diphosphonic acid), absolute changes in 31P-detectable high-energy phosphates in these extracts were determined and correlated with changes in bcl-2 protein levels, cell viability, cell cycle, apoptosis and total cellular glutathione (GSH) (an important defence against DNA damage from alkylating agents). After HN2 administration, bcl-2 protein levels in the HT-29 cell line rose at 2 h. Cell viability declined to 25% within 18 h, but apoptosis measured using fluorescence techniques remained in the 1-4% range. Increased cell division was noted at 4 h. Two high-energy interconvertible phosphates, NTP (P < or = 0.006) and phosphocreatine (PCr) (P < or = 0.0002), increased at 2 h concurrently with increased levels of bcl-2 protein and glutathione. This study demonstrates that bcl-2 and glutathione are up-regulated by HN2 and links this to a previously unexplained 31P MRS phenomenon: increased NTP after chemotherapy.
Full text
PDF


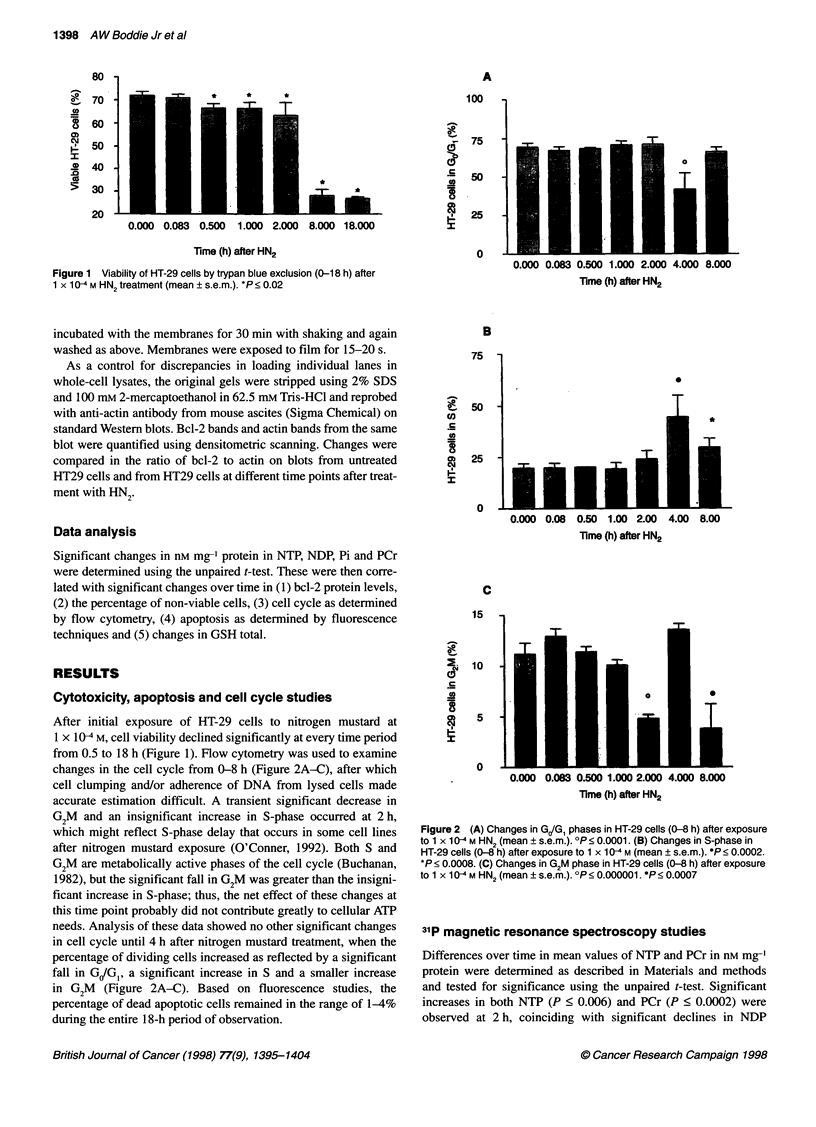
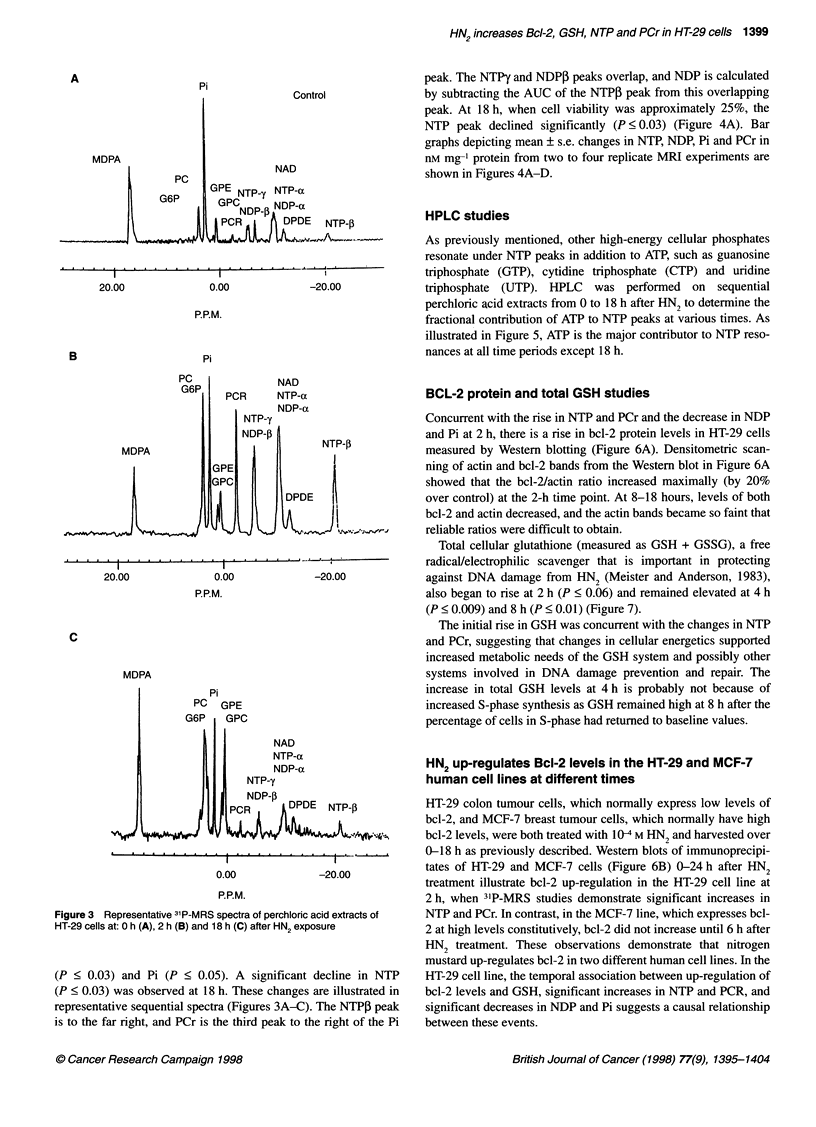
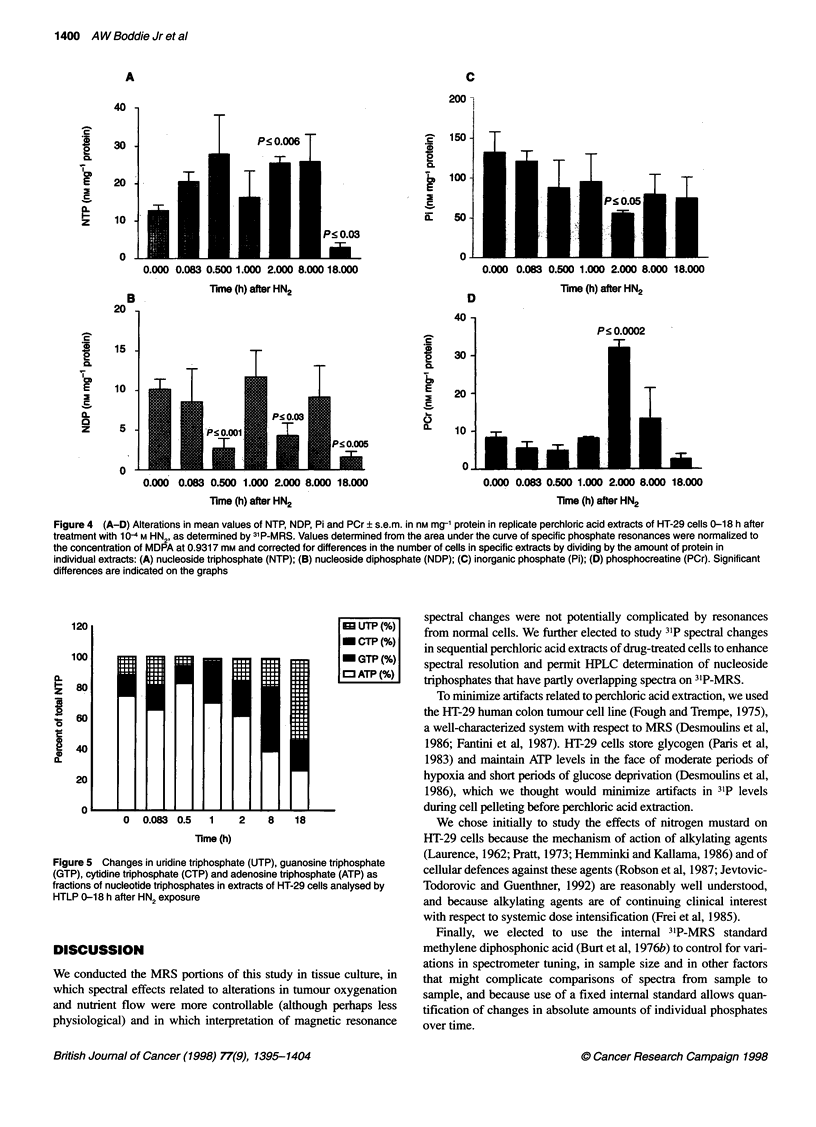



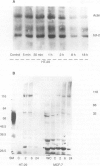
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- Armant M., Delespesse G., Sarfati M. IL-2 and IL-7 but not IL-12 protect natural killer cells from death by apoptosis and up-regulate bcl-2 expression. Immunology. 1995 Jun;85(2):331–337. [PMC free article] [PubMed] [Google Scholar]
- Berger N. A. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res. 1985 Jan;101(1):4–15. [PubMed] [Google Scholar]
- Berghmans K., Ruiz-Cabello J., Simpkins H., Andrews P. A., Cohen J. S. Increase in the ATP signal after treatment with cisplatin in two different cell lines studied by 31P NMR spectroscopy. Biochem Biophys Res Commun. 1992 Feb 28;183(1):114–120. doi: 10.1016/0006-291x(92)91616-x. [DOI] [PubMed] [Google Scholar]
- Bhaskar L., Mathan M. M., Balasubramanian K. A. Oxygen free radical-induced damage during colonic ischemia/reperfusion in rats. Mol Cell Biochem. 1995 Oct 4;151(1):9–14. doi: 10.1007/BF01076889. [DOI] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Bárány M., Glonek T. Phosphorus-31 nuclear magnetic resonance of contractile systems. Methods Enzymol. 1982;85(Pt B):624–676. doi: 10.1016/0076-6879(82)85055-6. [DOI] [PubMed] [Google Scholar]
- Cossarizza A., Kalashnikova G., Grassilli E., Chiappelli F., Salvioli S., Capri M., Barbieri D., Troiano L., Monti D., Franceschi C. Mitochondrial modifications during rat thymocyte apoptosis: a study at the single cell level. Exp Cell Res. 1994 Sep;214(1):323–330. doi: 10.1006/excr.1994.1264. [DOI] [PubMed] [Google Scholar]
- Decaudin D., Geley S., Hirsch T., Castedo M., Marchetti P., Macho A., Kofler R., Kroemer G. Bcl-2 and Bcl-XL antagonize the mitochondrial dysfunction preceding nuclear apoptosis induced by chemotherapeutic agents. Cancer Res. 1997 Jan 1;57(1):62–67. [PubMed] [Google Scholar]
- Deckwerth T. L., Johnson E. M., Jr Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol. 1993 Dec;123(5):1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulin F., Galons J. P., Canioni P., Marvaldi J., Cozzone P. J. 31P nuclear magnetic resonance study of a human colon adenocarcinoma cultured cell line. Cancer Res. 1986 Aug;46(8):3768–3774. [PubMed] [Google Scholar]
- Downes C. S., Ord M. J., Mullinger A. M., Collins A. R., Johnson R. T. Novobiocin inhibition of DNA excision repair may occur through effects on mitochondrial structure and ATP metabolism, not on repair topoisomerases. Carcinogenesis. 1985 Sep;6(9):1343–1352. doi: 10.1093/carcin/6.9.1343. [DOI] [PubMed] [Google Scholar]
- Eguchi Y., Shimizu S., Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997 May 15;57(10):1835–1840. [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Evanochko W. T., Sakai T. T., Ng T. C., Krishna N. R., Kim H. D., Zeidler R. B., Ghanta V. K., Brockman R. W., Schiffer L. M., Braunschweiger P. G. NMR study of in vivo RIF-1 tumors. Analysis of perchloric acid extracts and identification of 1H, 31P and 13C resonances. Biochim Biophys Acta. 1984 Sep 14;805(1):104–116. doi: 10.1016/0167-4889(84)90042-9. [DOI] [PubMed] [Google Scholar]
- Evelhoch J. L., Keller N. A., Corbett T. H. Response-specific adriamycin sensitivity markers provided by in vivo 31P nuclear magnetic resonance spectroscopy in murine mammary adenocarcinomas. Cancer Res. 1987 Jul 1;47(13):3396–3401. [PubMed] [Google Scholar]
- Fantini J., Galons J. P., Marvaldi J., Cozzone P. J., Canioni P. Growth of a human colonic adenocarcinoma cell line (HT 29) on microcarrier beads: metabolic studies by 31phosphorus nuclear magnetic resonance spectroscopy. Int J Cancer. 1987 Feb 15;39(2):255–260. doi: 10.1002/ijc.2910390222. [DOI] [PubMed] [Google Scholar]
- Frei E., 3rd, Cucchi C. A., Rosowsky A., Tantravahi R., Bernal S., Ervin T. J., Ruprecht R. M., Haseltine W. A. Alkylating agent resistance: in vitro studies with human cell lines. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2158–2162. doi: 10.1073/pnas.82.7.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman J., Hartl F. U. Principles of chaperone-assisted protein folding: differences between in vitro and in vivo mechanisms. Science. 1996 Jun 7;272(5267):1497–1502. doi: 10.1126/science.272.5267.1497. [DOI] [PubMed] [Google Scholar]
- Genestier L., Bonnefoy-Berard N., Rouault J. P., Flacher M., Revillard J. P. Tumor necrosis factor-alpha up-regulates Bcl-2 expression and decreases calcium-dependent apoptosis in human B cell lines. Int Immunol. 1995 Apr;7(4):533–540. doi: 10.1093/intimm/7.4.533. [DOI] [PubMed] [Google Scholar]
- Haldar S., Beatty C., Tsujimoto Y., Croce C. M. The bcl-2 gene encodes a novel G protein. Nature. 1989 Nov 9;342(6246):195–198. doi: 10.1038/342195a0. [DOI] [PubMed] [Google Scholar]
- Hemminki K., Kallama S. Reactions of nitrogen mustards with DNA. IARC Sci Publ. 1986;(78):55–70. [PubMed] [Google Scholar]
- Jevtović-Todorović V., Guenthner T. M. Depletion of a discrete nuclear glutathione pool by oxidative stress, but not by buthionine sulfoximine. Correlation with enhanced alkylating agent cytotoxicity to human melanoma cells in vitro. Biochem Pharmacol. 1992 Oct 6;44(7):1383–1393. doi: 10.1016/0006-2952(92)90540-y. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Desnoyers S., Ottaviano Y., Davidson N. E., Poirier G. G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993 Sep 1;53(17):3976–3985. [PubMed] [Google Scholar]
- Kluck R. M., Bossy-Wetzel E., Green D. R., Newmeyer D. D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997 Feb 21;275(5303):1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I. Membrane antioxidants. Ann N Y Acad Sci. 1988;551:17–33. doi: 10.1111/j.1749-6632.1988.tb22317.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lam M., Dubyak G., Chen L., Nuñez G., Miesfeld R. L., Distelhorst C. W. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow T., van Driel R., Bertram J. F., Strasser A. The protein product of the oncogene bcl-2 is a component of the nuclear envelope, the endoplasmic reticulum, and the outer mitochondrial membrane. Cell Growth Differ. 1994 Apr;5(4):411–417. [PubMed] [Google Scholar]
- Liu H., Lightfoot R., Stevens J. L. Activation of heat shock factor by alkylating agents is triggered by glutathione depletion and oxidation of protein thiols. J Biol Chem. 1996 Mar 1;271(9):4805–4812. [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Magnelli L., Cinelli M., Turchetti A., Chiarugi V. P. Bcl-2 overexpression abolishes early calcium waving preceding apoptosis in NIH-3T3 murine fibroblasts. Biochem Biophys Res Commun. 1994 Oct 14;204(1):84–90. doi: 10.1006/bbrc.1994.2429. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mills J. C., Nelson D., Erecińska M., Pittman R. N. Metabolic and energetic changes during apoptosis in neural cells. J Neurochem. 1995 Oct;65(4):1721–1730. doi: 10.1046/j.1471-4159.1995.65041721.x. [DOI] [PubMed] [Google Scholar]
- Murgia M., Pizzo P., Sandoná D., Zanovello P., Rizzuto R., Di Virgilio F. Mitochondrial DNA is not fragmented during apoptosis. J Biol Chem. 1992 Jun 5;267(16):10939–10941. [PubMed] [Google Scholar]
- Myers K. M., Fiskum G., Liu Y., Simmens S. J., Bredesen D. E., Murphy A. N. Bcl-2 protects neural cells from cyanide/aglycemia-induced lipid oxidation, mitochondrial injury, and loss of viability. J Neurochem. 1995 Dec;65(6):2432–2440. doi: 10.1046/j.1471-4159.1995.65062432.x. [DOI] [PubMed] [Google Scholar]
- Naruse S., Hirakawa K., Horikawa Y., Tanaka C., Higuchi T., Ueda S., Nishikawa H., Watari H. Measurements of in vivo 31P nuclear magnetic resonance spectra in neuroectodermal tumors for the evaluation of the effects of chemotherapy. Cancer Res. 1985 Jun;45(6):2429–2433. [PubMed] [Google Scholar]
- Neeman M., Eldar H., Rushkin E., Degani H. Chemotherapy-induced changes in the energetics of human breast cancer cells; 31P- and 13C-NMR studies. Biochim Biophys Acta. 1990 May 2;1052(2):255–263. doi: 10.1016/0167-4889(90)90219-4. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Orrenius S. Role of thiols in protection against biological reactive intermediates. Adv Exp Med Biol. 1986;197:41–51. doi: 10.1007/978-1-4684-5134-4_4. [DOI] [PubMed] [Google Scholar]
- O'Connor P. M., Ferris D. K., White G. A., Pines J., Hunter T., Longo D. L., Kohn K. W. Relationships between cdc2 kinase, DNA cross-linking, and cell cycle perturbations induced by nitrogen mustard. Cell Growth Differ. 1992 Jan;3(1):43–52. [PubMed] [Google Scholar]
- Ohta K., Iwai K., Kasahara Y., Taniguchi N., Krajewski S., Reed J. C., Miyawaki T. Immunoblot analysis of cellular expression of Bcl-2 family proteins, Bcl-2, Bax, Bcl-X and Mcl-1, in human peripheral blood and lymphoid tissues. Int Immunol. 1995 Nov;7(11):1817–1825. doi: 10.1093/intimm/7.11.1817. [DOI] [PubMed] [Google Scholar]
- Paris H., Terrain B., Viallard V., Rousset M., Zweibaum A., Murat J. C. Activity of glycogen metabolizing enzymes in glucose deprived HT 29 adenocarcinoma cell-line. Biochem Biophys Res Commun. 1983 Jan 27;110(2):371–377. doi: 10.1016/0006-291x(83)91158-0. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Robson C. N., Lewis A. D., Wolf C. R., Hayes J. D., Hall A., Proctor S. J., Harris A. L., Hickson I. D. Reduced levels of drug-induced DNA cross-linking in nitrogen mustard-resistant Chinese hamster ovary cells expressing elevated glutathione S-transferase activity. Cancer Res. 1987 Nov 15;47(22):6022–6027. [PubMed] [Google Scholar]
- Sakamuro D., Eviner V., Elliott K. J., Showe L., White E., Prendergast G. C. c-Myc induces apoptosis in epithelial cells by both p53-dependent and p53-independent mechanisms. Oncogene. 1995 Dec 7;11(11):2411–2418. [PubMed] [Google Scholar]
- Shidoji Y., Nakamura N., Moriwaki H., Muto Y. Rapid loss in the mitochondrial membrane potential during geranylgeranoic acid-induced apoptosis. Biochem Biophys Res Commun. 1997 Jan 3;230(1):58–63. doi: 10.1006/bbrc.1996.5883. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Eguchi Y., Kamiike W., Waguri S., Uchiyama Y., Matsuda H., Tsujimoto Y. Bcl-2 blocks loss of mitochondrial membrane potential while ICE inhibitors act at a different step during inhibition of death induced by respiratory chain inhibitors. Oncogene. 1996 Jul 4;13(1):21–29. [PubMed] [Google Scholar]
- Skulachev V. P. The laws of cell energetics. Eur J Biochem. 1992 Sep 1;208(2):203–209. doi: 10.1111/j.1432-1033.1992.tb17175.x. [DOI] [PubMed] [Google Scholar]
- Smets L. A., Van den Berg J., Acton D., Top B., Van Rooij H., Verwijs-Janssen M. BCL-2 expression and mitochondrial activity in leukemic cells with different sensitivity to glucocorticoid-induced apoptosis. Blood. 1994 Sep 1;84(5):1613–1619. [PubMed] [Google Scholar]
- Smith S. R., Martin P. A., Edwards R. H. Tumour pH and response to chemotherapy: an in vivo 31P magnetic resonance spectroscopy study in non-Hodgkin's lymphoma. Br J Radiol. 1991 Oct;64(766):923–928. doi: 10.1259/0007-1285-64-766-923. [DOI] [PubMed] [Google Scholar]
- Steen R. G. Response of solid tumors to chemotherapy monitored by in vivo 31P nuclear magnetic resonance spectroscopy: a review. Cancer Res. 1989 Aug 1;49(15):4075–4085. [PubMed] [Google Scholar]
- Strasser A., Anderson R. L. Bcl-2 and thermotolerance cooperate in cell survival. Cell Growth Differ. 1995 Jul;6(7):799–805. [PubMed] [Google Scholar]
- Tosi P., Visani G., Ottaviani E., Manfroi S., Tura S. In vitro culture with prednisolone increases BCL-2 protein expression in adult acute lymphoblastic leukemia cells. Am J Hematol. 1996 Apr;51(4):261–264. doi: 10.1002/(SICI)1096-8652(199604)51:4<261::AID-AJH2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Walajtys E. I., Gottesman D. P., Williamson J. R. Regulation of pyruvate dehydrogenase in rat liver mitochondria by phosphorylation-dephosphorylation. J Biol Chem. 1974 Mar 25;249(6):1857–1865. [PubMed] [Google Scholar]
- Walton M. I., Whysong D., O'Connor P. M., Hockenbery D., Korsmeyer S. J., Kohn K. W. Constitutive expression of human Bcl-2 modulates nitrogen mustard and camptothecin induced apoptosis. Cancer Res. 1993 Apr 15;53(8):1853–1861. [PubMed] [Google Scholar]
- Wehrle J. P., Li S. J., Rajan S. S., Steen R. G., Glickson J. D. 31P and 1H NMR spectroscopy of tumors in vivo: untreated growth and response to chemotherapy. Ann N Y Acad Sci. 1987;508:200–215. doi: 10.1111/j.1749-6632.1987.tb32905.x. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Owen C., Mela L., Weiner L. Control of mitochondrial respiration by the phosphate potential. Biochem Biophys Res Commun. 1973 Jul 2;53(1):326–333. doi: 10.1016/0006-291x(73)91437-x. [DOI] [PubMed] [Google Scholar]
- Yang J., Liu X., Bhalla K., Kim C. N., Ibrado A. M., Cai J., Peng T. I., Jones D. P., Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997 Feb 21;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Yao K. S., Clayton M., O'Dwyer P. J. Apoptosis in human adenocarcinoma HT29 cells induced by exposure to hypoxia. J Natl Cancer Inst. 1995 Jan 18;87(2):117–122. doi: 10.1093/jnci/87.2.117. [DOI] [PubMed] [Google Scholar]
- Yoon Y. S., Kim J. W., Kang K. W., Kim Y. S., Choi K. H., Joe C. O. Poly(ADP-ribosyl)ation of histone H1 correlates with internucleosomal DNA fragmentation during apoptosis. J Biol Chem. 1996 Apr 12;271(15):9129–9134. doi: 10.1074/jbc.271.15.9129. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Jacobus W. E., Korecky B., Brandejs-Barry Y. Bioenergetic consequences of cardiac phosphocreatine depletion induced by creatine analogue feeding. J Biol Chem. 1991 Oct 25;266(30):20296–20304. [PubMed] [Google Scholar]