Abstract
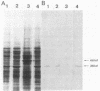
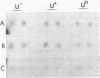
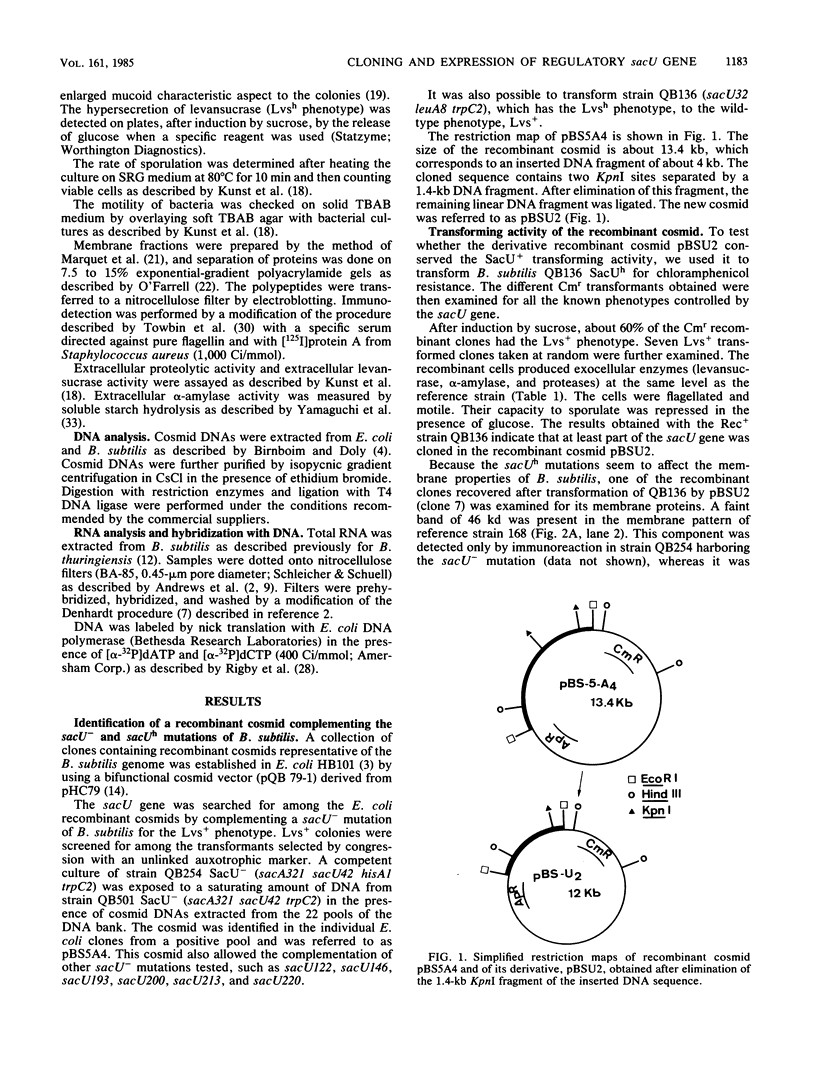
The regulatory wild-type locus sacU, which has a pleiotropic effect in Bacillus subtilis, notably on the synthesis of secreted proteins, was obtained from a colony bank of Escherichia coli harboring recombinant cosmids representative of the B. subtilis genome. It was shown that the sacU gene is located on a 2.4-kilobase KpnI-EcoRI fragment and that the cloned sequence is homologous to the corresponding chromosomal DNA fragment. The wild-type phenotype was recovered after transformation of SacU-, SacUh, and SacU- Rec- strains with the recombinant cosmid, indicating that the sacU locus has been cloned in totality. The sacU gene was expressed in a minicell-producing E. coli strain, and it was shown that it coded for a 46-kilodalton protein. In addition to the hypersecretion of proteins, SacUh mutants were characterized by the presence of a 46-kilodalton protein in the membrane fraction in higher amounts than were found in the wild-type strain. These mutants were also devoid of a 36-kilodalton polypeptide corresponding to the flagellin subunit. Analysis of the mRNA content of a secreted protein (levansucrase) in SacU- and SacUh mutants strongly suggested that the pleiotropic action of the sacU gene on the synthesis of levansucrase is exerted at a posttranscriptional level in B. subtilis cells and is probably correlated with the mechanism of secretion of exoenzymes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G. K., Dziadek M., Tamaoki T. Expression and methylation of the mouse alpha-fetoprotein gene in embryonic, adult, and neoplastic tissues. J Biol Chem. 1982 May 10;257(9):5148–5153. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Dedonder R. A., Lepesant J. A., Lepesant-Kejzlarová J., Billault A., Steinmetz M., Kunst F. Construction of a kit of reference strains for rapid genetic mapping in Bacillus subtilis 168. Appl Environ Microbiol. 1977 Apr;33(4):989–993. doi: 10.1128/aem.33.4.989-993.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Scher B., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: phenotypic characterization of radiation-sensitive recombination-deficient mutants. J Bacteriol. 1973 Apr;114(1):273–286. doi: 10.1128/jb.114.1.273-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziadek M. A., Andrews G. K. Tissue specificity of alpha-fetoprotein messenger RNA expression during mouse embryogenesis. EMBO J. 1983;2(4):549–554. doi: 10.1002/j.1460-2075.1983.tb01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouet A., Arnaud M., Klier A., Rapoport G. Characterization of the precursor form of the exocellular levansucrase from Bacillus subtilis. Biochem Biophys Res Commun. 1984 Mar 15;119(2):795–800. doi: 10.1016/s0006-291x(84)80320-4. [DOI] [PubMed] [Google Scholar]
- Fouet A., Klier A., Rapoport G. Cloning and expression in Escherichia coli of the sucrase gene from Bacillus subtilis. Mol Gen Genet. 1982;186(3):399–404. doi: 10.1007/BF00729460. [DOI] [PubMed] [Google Scholar]
- Glatron M. F., Rapoport G. Biosynthesis of the parasporal inclusion of Bacillus thuringiensis: half-life of its corresponding messenger RNA. Biochimie. 1972;54(10):1291–1301. doi: 10.1016/s0300-9084(72)80070-1. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Gabay J., Schwartz M. Evidence for a coupling of synthesis and export of an outer membrane protein in Escherichia coli. EMBO J. 1983;2(1):15–19. doi: 10.1002/j.1460-2075.1983.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Horiuchi S., Marty-Mazars D., Tai P. C., Davis B. D. Localization and quantitation of proteins characteristic of the complexed membrane of Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1215–1221. doi: 10.1128/jb.154.3.1215-1221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S., Tai P. C., Davis B. D. A 64-kilodalton membrane protein of Bacillus subtilis covered by secreting ribosomes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3287–3291. doi: 10.1073/pnas.80.11.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis M. A., Tokunaga M., Williams M. E., Loranger J. M., Chang S. Y., Chang S., Wu H. C. Nucleotide sequence of the Escherichia coli prolipoprotein signal peptidase (lsp) gene. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3708–3712. doi: 10.1073/pnas.81.12.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst F., Pascal M., Lepesant-Kejzlarova J., Lepesant J. A., Billault A., Dedonder R. Pleiotropic mutations affecting sporulation conditions and the syntheses of extracellular enzymes in Bacillus subtilis 168. Biochimie. 1974;56(11-12):1481–1489. doi: 10.1016/s0300-9084(75)80270-7. [DOI] [PubMed] [Google Scholar]
- Lepesant J. A., Kunst F., Lepesant-Kejzlarová J., Dedonder R. Chromosomal location of mutations affecting sucrose metabolism in Bacillus subtilis Marburg. Mol Gen Genet. 1972;118(2):135–160. doi: 10.1007/BF00267084. [DOI] [PubMed] [Google Scholar]
- Marquet M., Wagner M. C., Dedonder R. Separation of components of the phosphoenolpyruvate-glucose phosphotransferase system from Bacillus subtilis Marburg. Biochimie. 1971;53(10):1131–1134. doi: 10.1016/s0300-9084(71)80206-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Pascal M., Kunst F., Lepesant J. A., Dedonder R. Characterization of two sucrase activities in Bacillus subtilis Marburg. Biochimie. 1971;53(10):1059–1066. doi: 10.1016/s0300-9084(71)80193-1. [DOI] [PubMed] [Google Scholar]
- Petit-Glatron M. F., Chambert R. Levansucrase of Bacillus subtilis: Conclusive evidence that its production and export are unrelated to fatty-acid synthesis but modulated by membrane-modifying agents. Eur J Biochem. 1981 Oct;119(3):603–611. doi: 10.1111/j.1432-1033.1981.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Rambach A., Hogness D. S. Translation of Drosophila melanogaster sequences in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5041–5045. doi: 10.1073/pnas.74.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport G., Klier A., Billault A., Fargette F., Dedonder R. Construction of a colony bank of E. coli containing hybrid plasmids representative of the Bacillus subtilis 168 genome. Expression of functions harbored by the recombinant plasmids in B. subtilis. Mol Gen Genet. 1979 Oct 3;176(2):239–245. doi: 10.1007/BF00273218. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Kunst F., Dedonder R. Mapping of mutations affecting synthesis of exocellular enzymes in Bacillus subtilis. Identity of the sacUh, amyB and pap mutations. Mol Gen Genet. 1976 Nov 17;148(3):281–285. doi: 10.1007/BF00332902. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W. Bacterial leader peptidase, a membrane protein without a leader peptide, uses the same export pathway as pre-secretory proteins. Cell. 1984 Apr;36(4):1067–1072. doi: 10.1016/0092-8674(84)90056-4. [DOI] [PubMed] [Google Scholar]