Abstract
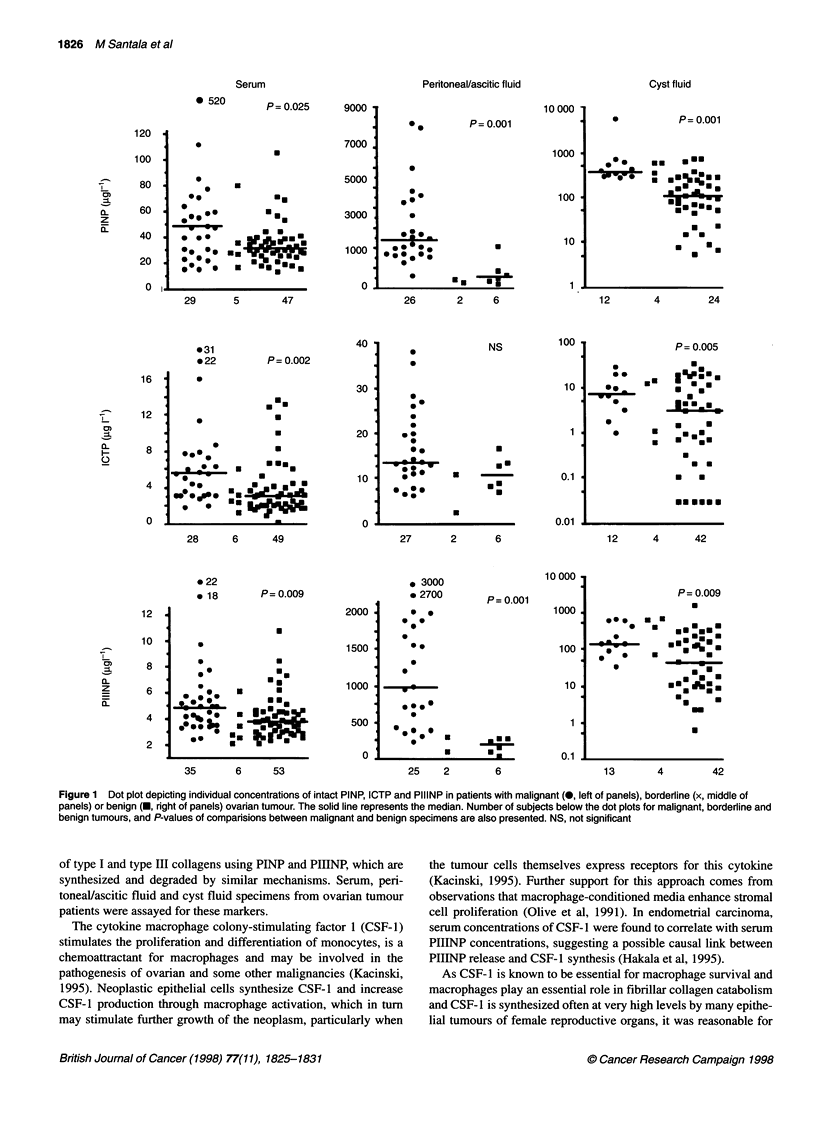
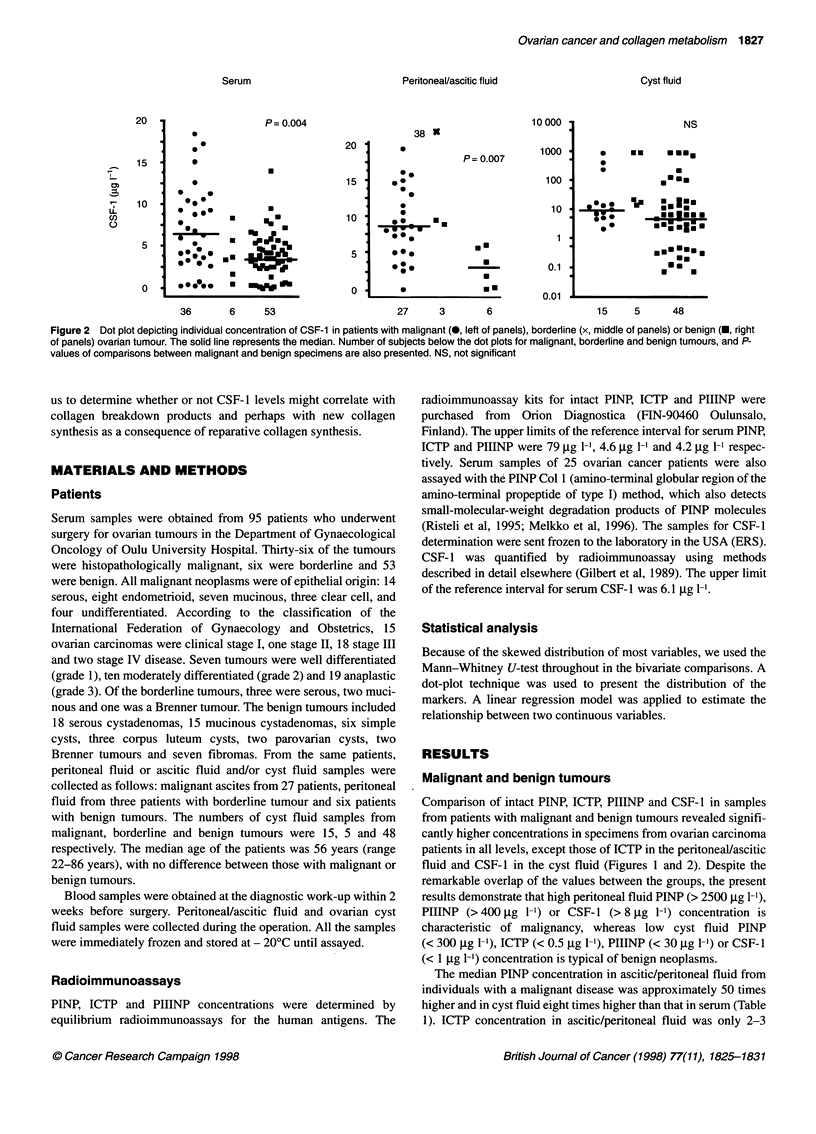
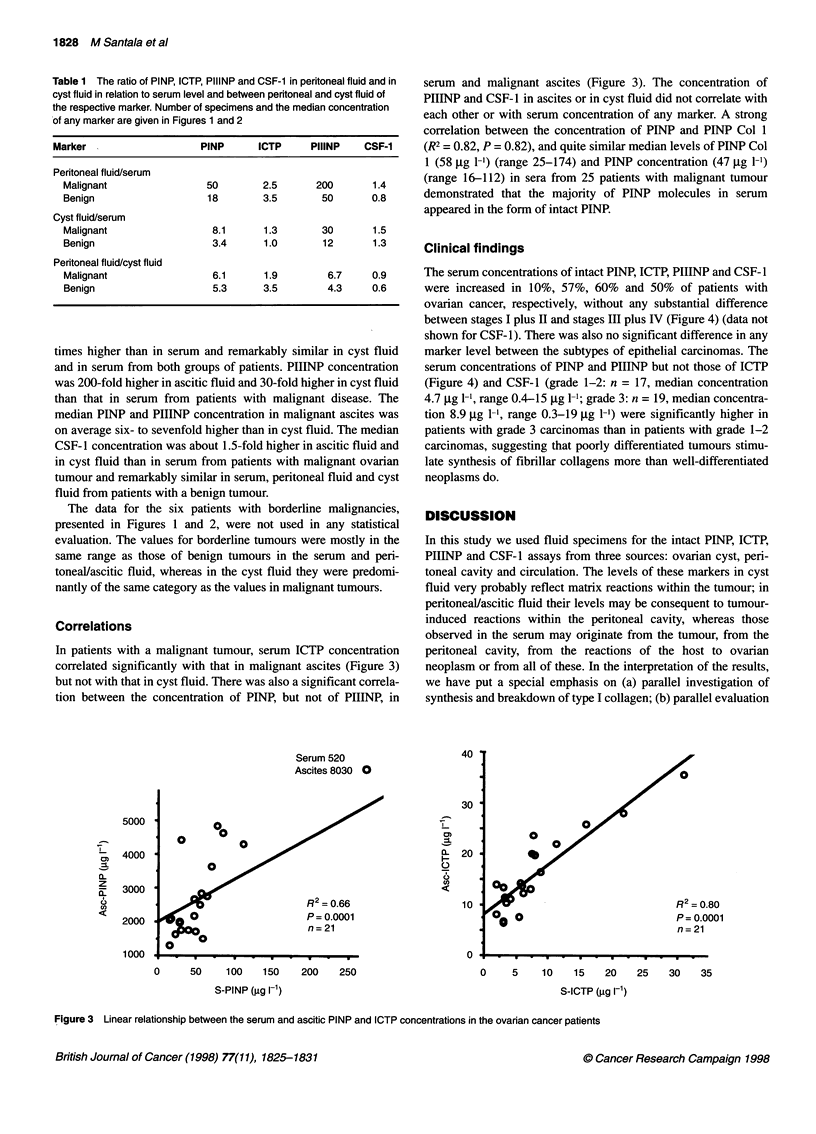
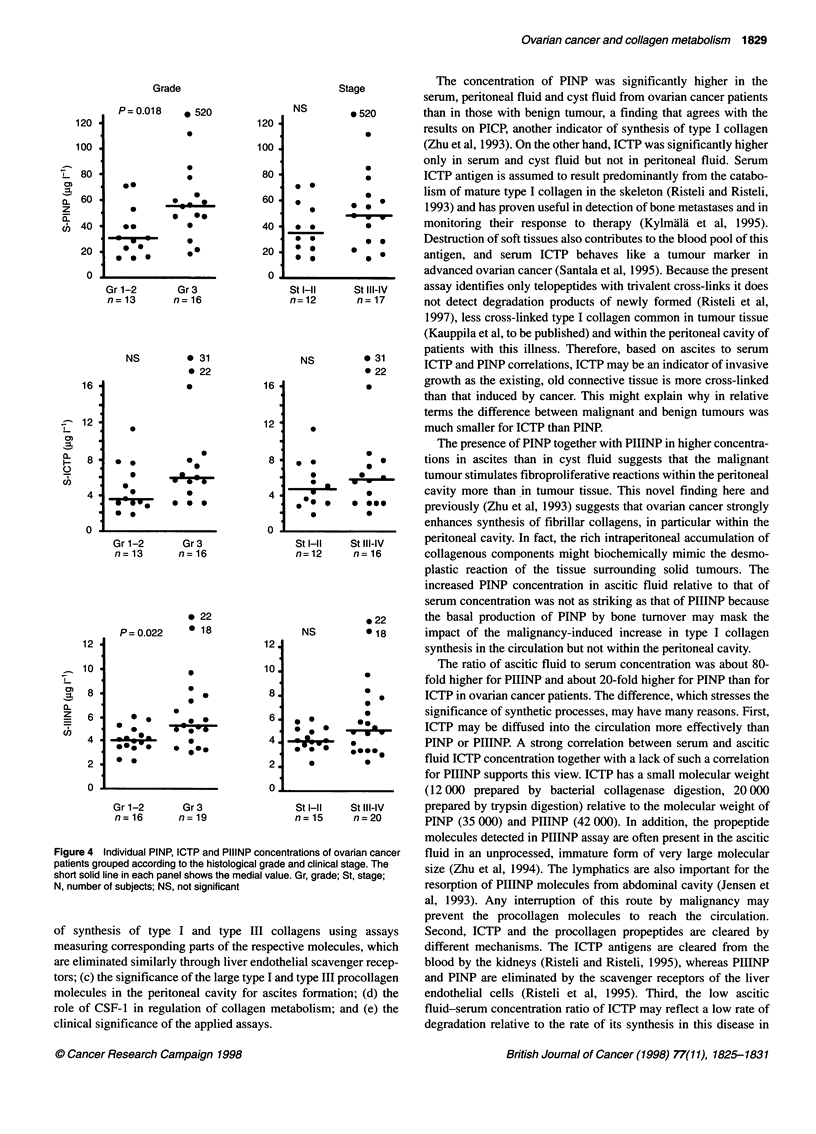
The synthesis and degradation of type I and type III interstitial collagens releases several antigenic metabolites, whose measurement allows the metabolism of connective tissue to be evaluated under a variety of different conditions. In this study we investigated the influence of benign and malignant ovarian neoplasms on the metabolism of these collagens. The study population comprised patients with benign (n = 53), borderline (n = 6) or malignant (n = 36) ovarian neoplasms. We quantified the serum, cyst fluid and peritoneal/ascitic fluid concentrations of the amino-terminal propeptide of type I (PINP) and III (PIIINP) procollagens, indicators of the synthesis of type I and III collagen, respectively and the cross-linked carboxy-terminal telopeptide of type I collagen (ICTP), an indicator of type I collagen degradation. Macrophage colony-stimulating factor 1 (CSF-1) concentration was also assayed as its serum level is increased in ovarian cancer and CSF-1 may be involved in the regulation of collagen metabolism. The concentration of each antigen was significantly higher in patients with malignant tumour than with benign neoplasm in each comparison, except for ICTP in peritoneal fluid and for CSF-1 in cyst fluid. The high ascitic fluid concentration of PINP, PIIINP or CSF-1 correlated with malignancy, and the low cyst fluid concentration of any of the four markers was indicative of benign tumour. Levels of CSF-1 did not correlate with the levels of any of the markers of collagen turnover. The concentration of PINP in ascites was about 50 times higher and in cyst fluid about eight times higher than that in the serum from patients with malignant tumour, whereas the respective ratios for ICTP were only 2.5 and 1.3. In such patients, the ratio of ascitic fluid to serum concentration was also about 80-fold higher for PIIINP and about 20-fold higher for PINP than for ICTP. The different distributions of PIIINP, PINP and ICTP suggests dominance of synthetic processes or retarded elimination of PIIINP and PINP in ovarian cancer. In advanced malignancies, the accumulation of PINP and PIIINP in abdominal space, possibly due to increased synthesis and/or failed resorption, may promote ascites formation. This study shows that both accelerated synthesis and breakdown of fibrillar collagens are characteristic of ovarian malignancy, and suggests that measurements of cyst fluid or ascitic fluid concentrations of collagen metabolites or CSF-1 could be used in the differential diagnosis of benign and malignant ovarian neoplasms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autio-Harmainen H., Karttunen T., Hurskainen T., Höyhtyä M., Kauppila A., Tryggvason K. Expression of 72 kilodalton type IV collagenase (gelatinase A) in benign and malignant ovarian tumors. Lab Invest. 1993 Sep;69(3):312–321. [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Timpl R. Collagen fibrillogenesis in human skin. Ann N Y Acad Sci. 1985;460:246–257. doi: 10.1111/j.1749-6632.1985.tb51172.x. [DOI] [PubMed] [Google Scholar]
- Gilbert H. S., Praloran V., Stanley E. R. Increased circulating CSF-1 (M-CSF) in myeloproliferative disease: association with myeloid metaplasia and peripheral bone marrow extension. Blood. 1989 Sep;74(4):1231–1234. [PubMed] [Google Scholar]
- Hakala A., Kacinski B. M., Stanley E. R., Kohorn E. I., Puistola U., Risteli J., Risteli L., Tomás C., Kauppila A. Macrophage colony-stimulating factor 1, a clinically useful tumor marker in endometrial adenocarcinoma: comparison with CA 125 and the aminoterminal propeptide of type III procollagen. Am J Obstet Gynecol. 1995 Jul;173(1):112–119. doi: 10.1016/0002-9378(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Janowska-Wieczorek A., Belch A. R., Jacobs A., Bowen D., Padua R. A., Paietta E., Stanley E. R. Increased circulating colony-stimulating factor-1 in patients with preleukemia, leukemia, and lymphoid malignancies. Blood. 1991 Apr 15;77(8):1796–1803. [PubMed] [Google Scholar]
- Jennings T. S., Dottino P. R., Mandeli J. P., Segna R. A., Kelliher K., Cohen C. J. Growth factor expression in normal peritoneum of patients with gynecologic carcinoma. Gynecol Oncol. 1994 Nov;55(2):190–197. doi: 10.1006/gyno.1994.1276. [DOI] [PubMed] [Google Scholar]
- Jensen L. T., Henriksen J. H., Olesen H. P., Risteli J., Lorenzen I. Lymphatic clearance of synovial fluid in conscious pigs: the aminoterminal propeptide of type III procollagen. Eur J Clin Invest. 1993 Dec;23(12):778–784. doi: 10.1111/j.1365-2362.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med. 1995 Feb;27(1):79–85. doi: 10.3109/07853899509031941. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M., Chambers S. K., Stanley E. R., Carter D., Tseng P., Scata K. A., Chang D. H., Pirro M. H., Nguyen J. T., Ariza A. The cytokine CSF-1 (M-CSF) expressed by endometrial carcinomas in vivo and in vitro, may also be a circulating tumor marker of neoplastic disease activity in endometrial carcinoma patients. Int J Radiat Oncol Biol Phys. 1990 Sep;19(3):619–626. doi: 10.1016/0360-3016(90)90488-6. [DOI] [PubMed] [Google Scholar]
- Kauppila A., Puistola U., Risteli J., Risteli L. Amino-terminal propeptide of type III procollagen: a new prognosis indicator in human ovarian cancer. Cancer Res. 1989 Apr 1;49(7):1885–1889. [PubMed] [Google Scholar]
- Kauppila S., Saarela J., Stenbäck F., Risteli J., Kauppila A., Risteli L. Expression of mRNAs for type I and type III procollagens in serous ovarian cystadenomas and cystadenocarcinomas. Am J Pathol. 1996 Feb;148(2):539–548. [PMC free article] [PubMed] [Google Scholar]
- Kylmälä T., Tammela T. L., Risteli L., Risteli J., Kontturi M., Elomaa I. Type I collagen degradation product (ICTP) gives information about the nature of bone metastases and has prognostic value in prostate cancer. Br J Cancer. 1995 May;71(5):1061–1064. doi: 10.1038/bjc.1995.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Melkko J., Kauppila S., Niemi S., Risteli L., Haukipuro K., Jukkola A., Risteli J. Immunoassay for intact amino-terminal propeptide of human type I procollagen. Clin Chem. 1996 Jun;42(6 Pt 1):947–954. [PubMed] [Google Scholar]
- Olive D. L., Montoya I., Riehl R. M., Schenken R. S. Macrophage-conditioned media enhance endometrial stromal cell proliferation in vitro. Am J Obstet Gynecol. 1991 Apr;164(4):953–958. [PubMed] [Google Scholar]
- Price F. V., Chambers S. K., Chambers J. T., Carcangiu M. L., Schwartz P. E., Kohorn E. I., Stanley E. R., Kacinski B. M. Colony-stimulating factor-1 in primary ascites of ovarian cancer is a significant predictor of survival. Am J Obstet Gynecol. 1993 Feb;168(2):520–527. doi: 10.1016/0002-9378(93)90485-2. [DOI] [PubMed] [Google Scholar]
- Risteli J., Niemi S., Kauppila S., Melkko J., Risteli L. Collagen propeptides as indicators of collagen assembly. Acta Orthop Scand Suppl. 1995 Oct;266:183–188. [PubMed] [Google Scholar]
- Risteli J., Niemi S., Trivedi P., Mäentausta O., Mowat A. P., Risteli L. Rapid equilibrium radioimmunoassay for the amino-terminal propeptide of human type III procollagen. Clin Chem. 1988 Apr;34(4):715–718. [PubMed] [Google Scholar]
- Risteli J., Risteli L. Analysing connective tissue metabolites in human serum. Biochemical, physiological and methodological aspects. J Hepatol. 1995;22(2 Suppl):77–81. [PubMed] [Google Scholar]
- Risteli L., Kauppila A., Mäkilä U. M., Risteli J. Aminoterminal propeptide of type-III procollagen in serum--an indicator of clinical behavior of advanced ovarian carcinoma? Int J Cancer. 1988 Mar 15;41(3):409–414. doi: 10.1002/ijc.2910410316. [DOI] [PubMed] [Google Scholar]
- Risteli L., Risteli J. Biochemical markers of bone metabolism. Ann Med. 1993 Aug;25(4):385–393. doi: 10.3109/07853899309147301. [DOI] [PubMed] [Google Scholar]
- Santala M., Risteli L., Puistola U., Risteli J., Kauppila A. Elevated serum ICTP concentrations reflect poor prognosis in patients with ovarian carcinoma. Ann Med. 1995 Feb;27(1):57–61. doi: 10.3109/07853899509031937. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hooff A. Connective tissue as an active participant in the process of malignant growth. Anticancer Res. 1986 Jul-Aug;6(4):775–780. [PubMed] [Google Scholar]
- Zhu G. G., Melkko J., Risteli J., Kauppila A., Risteli L. Differential processing of type I and type III procollagens in the tumour cysts and peritoneal ascitic fluid of patients with benign and malignant ovarian tumours. Clin Chim Acta. 1994 Sep;229(1-2):87–97. doi: 10.1016/0009-8981(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Zhu G. G., Risteli J., Puistola U., Kauppila A., Risteli L. Progressive ovarian carcinoma induces synthesis of type I and type III procollagens in the tumor tissue and peritoneal cavity. Cancer Res. 1993 Oct 15;53(20):5028–5032. [PubMed] [Google Scholar]
- Zhu G. G., Risteli L., Mäkinen M., Risteli J., Kauppila A., Stenbäck F. Immunohistochemical study of type I collagen and type I pN-collagen in benign and malignant ovarian neoplasms. Cancer. 1995 Feb 15;75(4):1010–1017. doi: 10.1002/1097-0142(19950215)75:4<1010::aid-cncr2820750417>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]


