Abstract
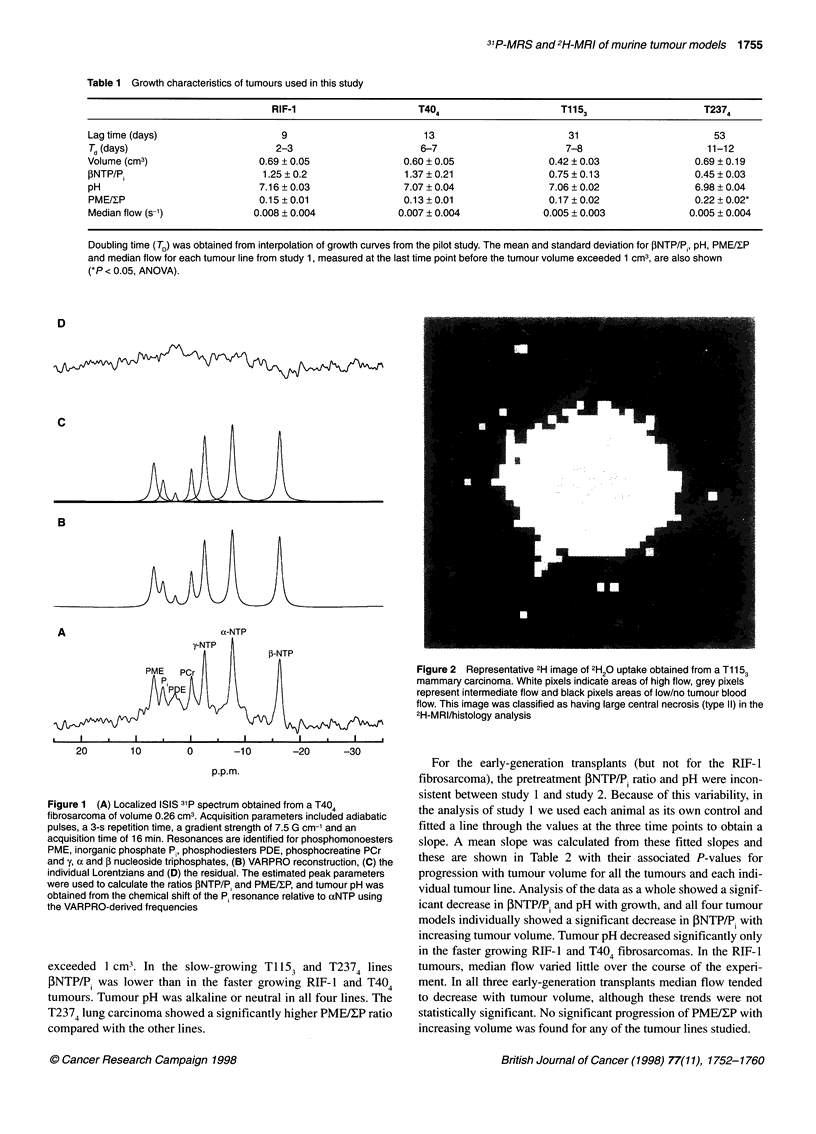
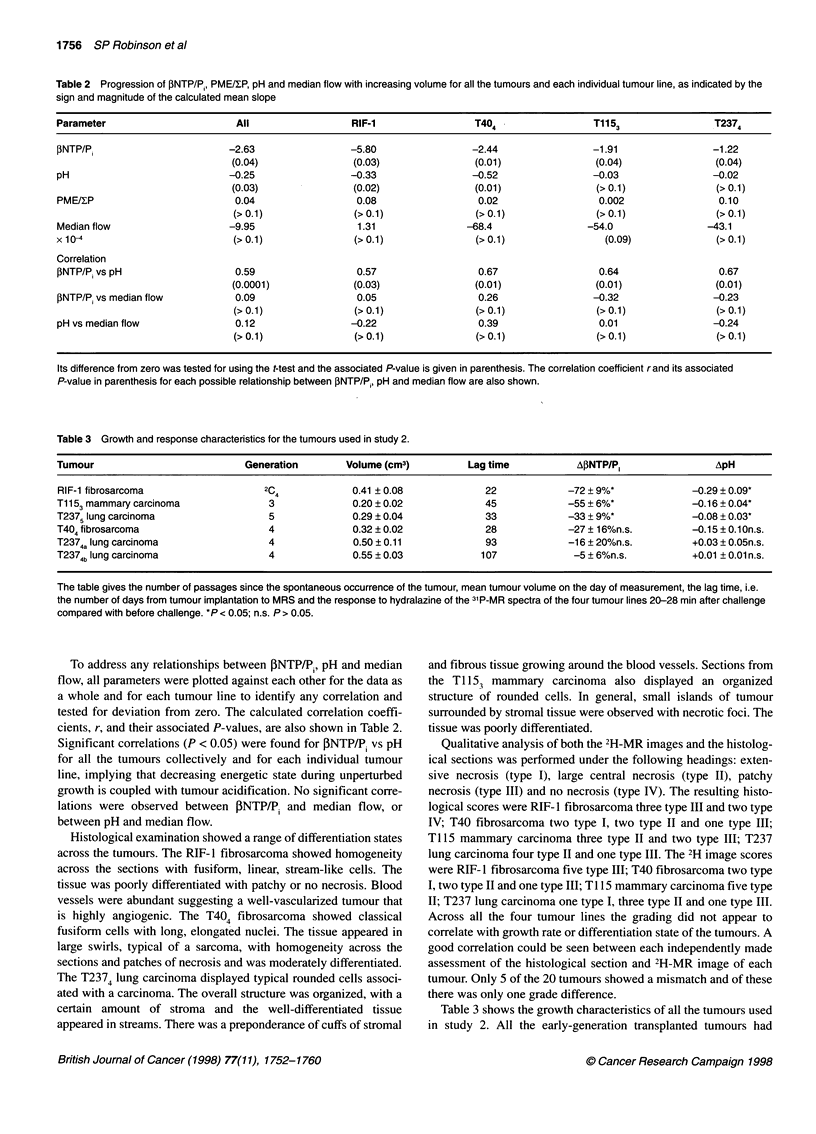
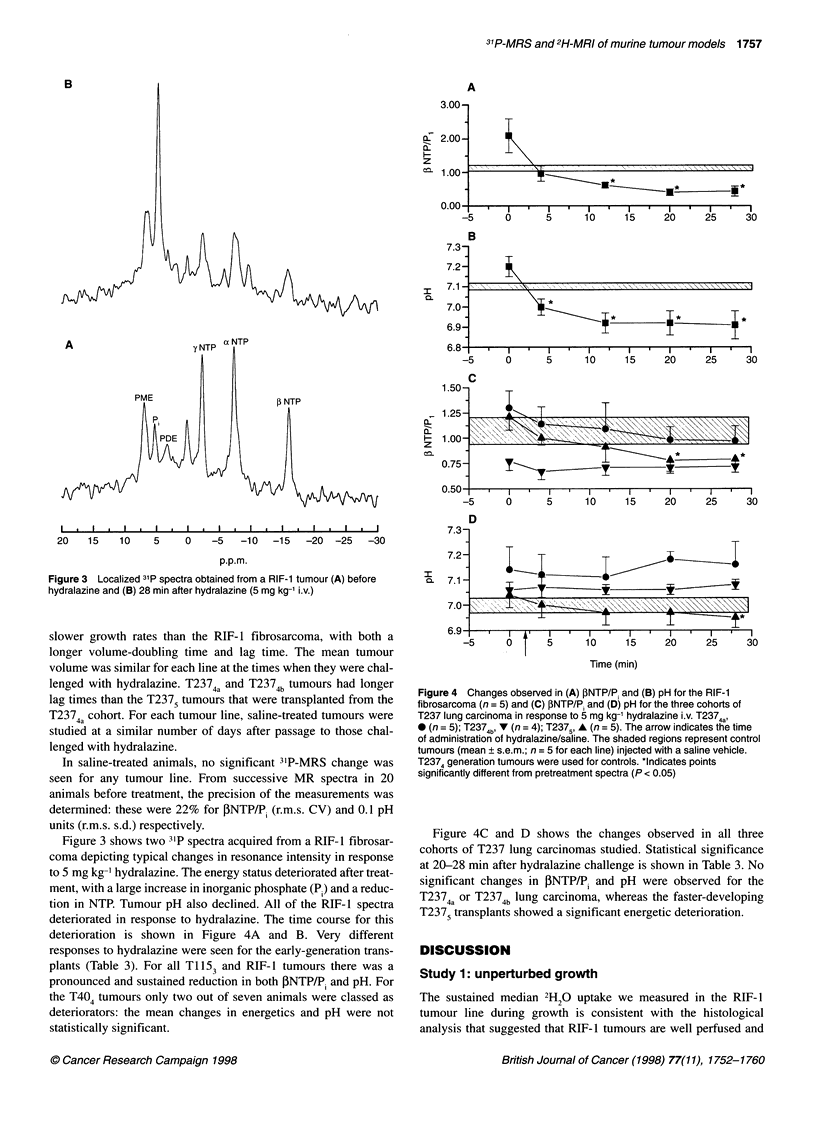
The objective of this study was first to determine whether three slowly growing early-generation murine transplantable tumours, the T40 fibrosarcoma, T115 mammary carcinoma and T237 lung carcinoma, exhibit patterns of energetics and blood flow during growth that are different from those of the faster growing RIF-1 fibrosarcoma. Serial measurements were made with 31P-magnetic resonance spectroscopy (MRS), relating to nutritive blood flow and 2H-magnetic resonance imaging (MRI), which is sensitive to both nutritive and large-vessel (non-nutritive) flow. All four tumour lines showed a decrease in betaNTP/Pi and pH with growth; however, each line showed a different pattern of blood flow that did not correlate with the decrease in energetics. Qualitative histological analysis strongly correlated with the 2H-MRI. Second, their response to 5 mg kg(-1) hydralazine i.v. was monitored by 31P-MRS. A marked decrease in betaNTP/Pi and pH was observed in both the RIF-1 fibrosarcoma and the third-generation T115 mammary carcinoma after hydralazine challenge. In contrast, the fourth generation T40 fibrosarcoma and T237 lung carcinoma showed no change in 31P-MRS parameters. However, a fifth-generation T237 cohort, which grew approximately three times faster than fourth-generation T237 cohorts, exhibited a significant deterioration in betaNTP/Pi and pH in response to hydralazine. These data are consistent with a decoupling between large-vessel and nutritive blood flow and indicate that early-generation transplants that have a slow growth rate and vascular tone are more appropriate models of human tumour vasculature than more rapidly growing, repeatedly transplanted tumours.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhujwalla Z. M., Tozer G. M., Field S. B., Maxwell R. J., Griffiths J. R. The energy metabolism of RIF-1 tumours following hydralazine. Radiother Oncol. 1990 Nov;19(3):281–291. doi: 10.1016/0167-8140(90)90155-p. [DOI] [PubMed] [Google Scholar]
- Bhujwalla Z. M., Tozer G. M., Field S. B., Proctor E., Busza A., Williams S. R. The combined measurement of blood flow and metabolism in RIF-1 tumours in vivo. A study using H2 flow and 31P NMR spectroscopy. NMR Biomed. 1990 Aug;3(4):178–183. doi: 10.1002/nbm.1940030406. [DOI] [PubMed] [Google Scholar]
- Burney I. A., Maxwell R. J., Griffiths J. R., Field S. B. Deuterium nuclear magnetic resonance imaging of the developmental pattern of tumour blood flow. EXS. 1992;61:357–361. doi: 10.1007/978-3-0348-7001-6_57. [DOI] [PubMed] [Google Scholar]
- Denekamp J. The choice of experimental models in cancer research: the key to ultimate success or failure? NMR Biomed. 1992 Sep-Oct;5(5):234–237. doi: 10.1002/nbm.1940050507. [DOI] [PubMed] [Google Scholar]
- Dunn J. F., Frostick S., Adams G. E., Stratford I. J., Howells N., Hogan G., Radda G. K. Induction of tumour hypoxia by a vasoactive agent. A combined NMR and radiobiological study. FEBS Lett. 1989 Jun 5;249(2):343–347. doi: 10.1016/0014-5793(89)80655-6. [DOI] [PubMed] [Google Scholar]
- Evanochko W. T., Sakai T. T., Ng T. C., Krishna N. R., Kim H. D., Zeidler R. B., Ghanta V. K., Brockman R. W., Schiffer L. M., Braunschweiger P. G. NMR study of in vivo RIF-1 tumors. Analysis of perchloric acid extracts and identification of 1H, 31P and 13C resonances. Biochim Biophys Acta. 1984 Sep 14;805(1):104–116. doi: 10.1016/0167-4889(84)90042-9. [DOI] [PubMed] [Google Scholar]
- Evelhoch J. L., Sapareto S. A., Nussbaum G. H., Ackerman J. J. Correlations between 31P NMR spectroscopy and 15O perfusion measurements in the RIF-1 murine tumor in vivo. Radiat Res. 1986 Apr;106(1):122–131. [PubMed] [Google Scholar]
- Falk P. Differences in vascular pattern between the spontaneous and the transplanted C3H mouse mammary carcinoma. Eur J Cancer Clin Oncol. 1982 Feb;18(2):155–165. doi: 10.1016/0277-5379(82)90059-1. [DOI] [PubMed] [Google Scholar]
- Field S. B., Needham S., Burney I. A., Maxwell R. J., Coggle J. E., Griffiths J. R. Differences in vascular response between primary and transplanted tumours. Br J Cancer. 1991 May;63(5):723–726. doi: 10.1038/bjc.1991.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J. R., Cady E., Edwards R. H., McCready V. R., Wilkie D. R., Wiltshaw E. 31P-NMR studies of a human tumour in situ. Lancet. 1983 Jun 25;1(8339):1435–1436. doi: 10.1016/s0140-6736(83)92375-9. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Christensen K. L., Overgaard J. Relationship between the hydralazine-induced changes in murine tumor blood supply and mouse blood pressure. Int J Radiat Oncol Biol Phys. 1992;22(3):455–458. doi: 10.1016/0360-3016(92)90852-9. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- Jirtle R. L. Chemical modification of tumour blood flow. Int J Hyperthermia. 1988 Jul-Aug;4(4):355–371. doi: 10.3109/02656738809016490. [DOI] [PubMed] [Google Scholar]
- Kalmus J., Okunieff P., Vaupel P. Dose-dependent effects of hydralazine on microcirculatory function and hyperthermic response of murine FSall tumors. Cancer Res. 1990 Jan 1;50(1):15–19. [PubMed] [Google Scholar]
- Larcombe-McDouall J. B., Mattiello J., McCoy C. L., Simpson N. E., Seyedsadr M., Evelhoch J. L. Size dependence of regional blood flow in murine tumours using deuterium magnetic resonance imaging. Int J Radiat Biol. 1991 Jul-Aug;60(1-2):109–113. doi: 10.1080/09553009114551651. [DOI] [PubMed] [Google Scholar]
- Lilly M. B., Katholi C. R., Ng T. C. Direct relationship between high-energy phosphate content and blood flow in thermally treated murine tumors. J Natl Cancer Inst. 1985 Nov;75(5):885–889. doi: 10.1093/jnci/75.5.885. [DOI] [PubMed] [Google Scholar]
- Maxwell R. J., Workman P., Griffiths J. R. Demonstration of tumor-selective retention of fluorinated nitroimidazole probes by 19F magnetic resonance spectroscopy in vivo. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):925–929. doi: 10.1016/0360-3016(89)90888-2. [DOI] [PubMed] [Google Scholar]
- McCredie J. A., Inch W. R., Sutherland R. M. Differences in growth and morphology between the spontaneous C3H mammary carcinoma in the mouse and its syngeneic transplants. Cancer. 1971 Mar;27(3):635–642. doi: 10.1002/1097-0142(197103)27:3<635::aid-cncr2820270319>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Menke H., Vaupel P. Effect of injectable or inhalational anesthetics and of neuroleptic, neuroleptanalgesic, and sedative agents on tumor blood flow. Radiat Res. 1988 Apr;114(1):64–76. [PubMed] [Google Scholar]
- Moulder J. E., Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984 May;10(5):695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Negendank W. Studies of human tumors by MRS: a review. NMR Biomed. 1992 Sep-Oct;5(5):303–324. doi: 10.1002/nbm.1940050518. [DOI] [PubMed] [Google Scholar]
- Nordsmark M., Maxwell R. J., Wood P. J., Stratford I. J., Adams G. E., Overgaard J., Horsman M. R. Effect of hydralazine in spontaneous tumours assessed by oxygen electrodes and 31P-magnetic resonance spectroscopy. Br J Cancer Suppl. 1996 Jul;27:S232–S235. [PMC free article] [PubMed] [Google Scholar]
- Okunieff P. G., Koutcher J. A., Gerweck L., McFarland E., Hitzig B., Urano M., Brady T., Neuringer L., Suit H. D. Tumor size dependent changes in a murine fibrosarcoma: use of in vivo 31P NMR for non-invasive evaluation of tumor metabolic status. Int J Radiat Oncol Biol Phys. 1986 May;12(5):793–799. doi: 10.1016/0360-3016(86)90038-6. [DOI] [PubMed] [Google Scholar]
- Okunieff P., Kallinowski F., Vaupel P., Neuringer L. J. Effects of hydralazine-induced vasodilation on the energy metabolism of murine tumors studied by in vivo 31P-nuclear magnetic resonance spectroscopy. J Natl Cancer Inst. 1988 Jul 20;80(10):745–750. doi: 10.1093/jnci/80.10.745. [DOI] [PubMed] [Google Scholar]
- Prichard J. W., Alger J. R., Behar K. L., Petroff O. A., Shulman R. G. Cerebral metabolic studies in vivo by 31P NMR. Proc Natl Acad Sci U S A. 1983 May;80(9):2748–2751. doi: 10.1073/pnas.80.9.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofstad E. K., Howell R. L., DeMuth P., Ceckler T. L., Sutherland R. M. 31P NMR spectroscopy in vivo of two murine tumor lines with widely different fractions of radiobiologically hypoxic cells. Int J Radiat Biol. 1988 Oct;54(4):635–649. doi: 10.1080/09553008814552071. [DOI] [PubMed] [Google Scholar]
- Rowell N. P., Flower M. A., McCready V. R., Cronin B., Horwich A. The effects of single dose oral hydralazine on blood flow through human lung tumours. Radiother Oncol. 1990 Aug;18(4):283–292. doi: 10.1016/0167-8140(90)90108-9. [DOI] [PubMed] [Google Scholar]
- Sansom J. M., Wood P. J. 31P MRS of tumour metabolism in anaesthetized vs conscious mice. NMR Biomed. 1994 Jun;7(4):167–171. doi: 10.1002/nbm.1940070403. [DOI] [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozer G. M., Bhujwalla Z. M., Griffiths J. R., Maxwell R. J. Phosphorus-31 magnetic resonance spectroscopy and blood perfusion of the RIF-1 tumor following X-irradiation. Int J Radiat Oncol Biol Phys. 1989 Jan;16(1):155–164. doi: 10.1016/0360-3016(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Vaupel P., Okunieff P., Kallinowski F., Neuringer L. J. Correlations between 31P-NMR spectroscopy and tissue O2 tension measurements in a murine fibrosarcoma. Radiat Res. 1989 Dec;120(3):477–493. [PubMed] [Google Scholar]
- Vaupel P., Schaefer C., Okunieff P. Intracellular acidosis in murine fibrosarcomas coincides with ATP depletion, hypoxia, and high levels of lactate and total Pi. NMR Biomed. 1994 May;7(3):128–136. doi: 10.1002/nbm.1940070305. [DOI] [PubMed] [Google Scholar]
- Wood P. J., Stratford I. J., Sansom J. M., Cattanach B. M., Quinney R. M., Adams G. E. The response of spontaneous and transplantable murine tumors to vasoactive agents measured by 31P magnetic resonance spectroscopy. Int J Radiat Oncol Biol Phys. 1992;22(3):473–476. doi: 10.1016/0360-3016(92)90856-d. [DOI] [PubMed] [Google Scholar]
- van den Boogaart A., Howe F. A., Rodrigues L. M., Stubbs M., Griffiths J. R. In vivo 31P MRS: absolute concentrations, signal-to-noise and prior knowledge. NMR Biomed. 1995 Apr;8(2):87–93. doi: 10.1002/nbm.1940080207. [DOI] [PubMed] [Google Scholar]
- van der Veen J. W., de Beer R., Luyten P. R., van Ormondt D. Accurate quantification of in vivo 31P NMR signals using the variable projection method and prior knowledge. Magn Reson Med. 1988 Jan;6(1):92–98. doi: 10.1002/mrm.1910060111. [DOI] [PubMed] [Google Scholar]



